Abstract
Introduction Vital pulp therapy (VPT) offers a biologically driven, conservative alternative to root canal treatment, aiming to preserve pulp vitality. Hydrogel scaffolds have gained attention as a regenerative strategy due to their tissue healing and bioactive properties. However, a comprehensive synthesis of their role in pulp regeneration remains limited.
Aim This systematic review explored the current evidence on applying hydrogel scaffolds in VPT and their potential to support pulp regeneration and healing.
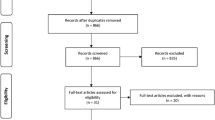
Methods A structured literature search was conducted across PubMed, ScienceDirect, Cochrane Library, Google Scholar, and EBSCO, targeting randomised controlled trials published between 2014 and 2024. The studies evaluated the effectiveness of hydrogel scaffolds over conventional materials in pulp regeneration therapy. Study quality was assessed using RoB2 to evaluate the risk of bias.
Results In total, 336 studies were yielded, while nine were included after screening. Hydrogel-based scaffolds were compared to non-hydrogel-based scaffolds.
Discussion Findings suggest that hydrogel-based scaffolds significantly enhance pulp regeneration, promoting tissue formation, reducing inflammatory cell infiltration, and preventing root resorption.
Conclusion Hydrogel-based scaffolds offer significant advantages in VPT compared to non-hydrogel-based scaffolds; however, further research is needed to identify optimal hydrogel types and delivery methods and to develop standardised long-term protocols.
Key points
-
Hydrogel scaffolds significantly enhance pulp regeneration by promoting tissue formation, reducing inflammation, and preventing root resorption in vital pulp therapy.
-
Hydrogel-based scaffolds offer superior biological performance compared to conventional materials due to their biomimetic and bioactive properties.
-
Despite promising results, further research is needed to identify optimal hydrogel formulations and delivery methods, and to establish standardised clinical protocols for long-term success.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
This study is a systematic review and does not involve generating or analysing primary data. All data supporting the findings of this review are derived from previously published studies, which are cited within the manuscript. Further details can be obtained from the corresponding author upon reasonable request.
References
França C M, Riggers R, Muschler J L et al. 3D-imaging of whole neuronal and vascular networks of the human dental pulp via clarity and light sheet microscopy. Sci Rep 2019; 9: 10860.
Edwards D, Rasaiah S, Hamzah Ahmed S et al. The financial and quality of life impact of urgent dental presentations: a cross-sectional study. Int Endod J 2023; 56: 697–709.
Pohl S, Akamp T, Smeda M et al. Understanding dental pulp inflammation: from signaling to structure. Front Immunol 2024; 15: 1474466.
Yu C, Abbott P V. An overview of the dental pulp: its functions and responses to injury. Aust Dent J 2007; DOI: 10.1111/j.1834-7819.2007.tb00525.x.
Wolters W J, Duncan H F, Tomson P L et al. Minimally invasive endodontics: a new diagnostic system for assessing pulpitis and subsequent treatment needs. Int Endod J 2017; 50: 825–829.
Touré B, Faye B, Kane A W, Lo C M, Niang B, Boucher Y. Analysis of reasons for extraction of endodontically treated teeth: a prospective study. J Endod 2011; 37: 1512–1515.
Duncan H F, Galler K M, Tomson P L et al. European Society of Endodontology position statement: management of deep caries and the exposed pulp. Int Endod J 2019; 52: 923–934.
Yan H, De Deus G, Kristoffersen I M et al. Regenerative endodontics by cell homing: a review of recent clinical trials. J Endod 2023; 49: 4–17.
Xie Z, Shen Z, Zhan P et al. Functional dental pulp regeneration: basic research and clinical translation. Int J Mol Sci 2021; 22: 8991.
Tong H J, Rajan S, Bhujel N, Kang J, Duggal M, Nazzal H. Regenerative endodontic therapy in the management of nonvital immature permanent teeth: a systematic review-outcome evaluation and meta-analysis. J Endod 2017; 43: 1453–1464.
Galler K M, Krastl G, Simon S et al. European Society of Endodontology position statement: Revitalization procedures. Int Endod J 2016; 49: 717–723.
Song J, Gerecht S. Hydrogels to recapture extracellular matrix cues that regulate vascularization. Arterioscler Thromb Vasc Biol 2023; DOI: 10.1161/ATVBAHA.122.318235.
Abbass M M S, El-Rashidy A A, Sadek K M et al. Hydrogels and dentin-pulp complex regeneration: from the benchtop to clinical translation. Polymers 2020; 12: 2935.
Sordi M B, Cruz A, Fredel M C, Magini R, Sharpe P T. Three-dimensional bioactive hydrogel-based scaffolds for bone regeneration in implant dentistry. Mater Sci Eng 2021; 124: 112055.
Phan C M, Luu C H, Murugesan M et al. Injectable gelatin-pectin hydrogel for dental tissue engineering: Enhanced angiogenesis and antibacterial efficacy for pulpitis therapy. Int J Biol Macromol 2025; 284: 137939.
Xie Z, Jiang W, Liu H et al. Antimicrobial peptide- and dentin matrix-functionalized hydrogel for vital pulp therapy via synergistic bacteriostasis, immunomodulation, and dentinogenesis. Adv Healthc Mater 2024; 13: 2303709.
Page M J, McKenzie J E, Bossuyt P M et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; DOI: 10.1136/bmj.n71.
Sterne J A C, Savović J, Page M J et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; DOI: 10.1136/bmj.l4898.
Charrois T L. Systematic reviews: what do you need to know to get started? Can J Hosp Pharm 2015; 68: 144–148.
Elnawam H, Thabet A, Mobarak A et al. Bovine pulp extracellular matrix hydrogel for regenerative endodontic applications: in vitro characterization and in vivo analysis in a necrotic tooth model. Head Face Med 2024; 20: 61.
Holiel A A, Mahmoud E M, Abdel-Fattah W M. Tomographic evaluation of direct pulp capping using a novel injectable treated dentin matrix hydrogel: a 2-year randomized controlled clinical trial. Clin Oral Investig 2021; 25: 4621–4634.
Sedek E M, Abdelkader S, Fahmy A E, Kamoun E A, Nouh S R, Khalil N M. Histological evaluation of the regenerative potential of a novel photocrosslinkable gelatin-treated dentin matrix hydrogel in direct pulp capping: an animal study. BMC Oral Health 2024; 24: 114.
Jang J-H, Moon J-H, Kim S G, Kim S-Y. Pulp regeneration with hemostatic matrices as a scaffold in an immature tooth minipig model. Sci Rep 2020; 10: 12536.
Zhang X, Zhou X, Zhai W et al. Novel L-(CaP-ZnP)/SA nanocomposite hydrogel with dual anti-inflammatory and mineralization effects for efficient vital pulp therapy. Int J Nanomed 2024; 19: 6659–6676.
Holiel A A, Mahmoud E M, Abdel-Fattah W M, Kawana K Y. Histological evaluation of the regenerative potential of a novel treated dentin matrix hydrogel in direct pulp capping. Clin Oral Investig 2021; 25: 2101–2112.
Zhou L, Shi W, Zhang X et al. Injectable tannin-containing hydroxypropyl chitin hydrogel as novel bioactive pulp capping material accelerates repair of inflamed dental pulp. Biomolecules 2024; 14: 1129.
Abdelsalam M S, Elgendy A A, Abu-Seida A M, Abdelaziz T M, Issa M H, El-Haddad K E. Histological evaluation of the regenerative potential of injectable hyaluronic acid hydrogel or collagen with blood clot as scaffolds during revascularization of immature necrotic dog's teeth. Open Vet J 2024; 14: 3004–3016.
Xia K, Chen Z, Chen J et al. RGD- and VEGF-mimetic peptide epitope-functionalized self-assembling peptide hydrogels promote dentin-pulp complex regeneration. Int J Nanomed 2020; 15: 6631–6647.
Sarfi S, Azaryan E, Naseri M. Immune system of dental pulp in inflamed and normal tissue. DNA Cell Biol 2024; 43: 369–386.
Han Y, Xu J, Chopra H et al. Injectable tissue-specific hydrogel system for pulp-dentin regeneration. J Dent Res 2024; 103: 398–408.
Ahmad Z, Salman S, Khan S A et al. Versatility of hydrogels: from synthetic strategies, classification, and properties to biomedical applications. Gels 2022; 8: 167.
Ho T-C, Chang C-C, Chan H-P et al. Hydrogels: properties and applications in biomedicine. Molecules 2022; 27: 2902.
Quigley R M, Kearney M, Kennedy O D, Duncan H F. Tissue engineering approaches for dental pulp regeneration: The development of novel bioactive materials using pharmacological epigenetic inhibitors. Bioactive Mater 2024; 40: 182–211.
Han B, Cao C, Wang A et al. Injectable double-network hydrogel-based three-dimensional cell culture systems for regenerating dental pulp. ACS Appl Mater Interfaces 2023; 15: 7821–7832.
Lambricht L, De Berdt P, Vanacker J et al. The type and composition of alginate and hyaluronic-based hydrogels influence the viability of stem cells of the apical papilla. Dent Mater 2014; DOI: 10.1016/j.dental.2014.08.369.
Žigon-Branc S, Markovic M, Van Hoorick J et al. Impact of hydrogel stiffness on differentiation of human adipose-derived stem cell microspheroids. Tissue Eng Part A 2019; 25: 1369–1380.
Ghandforoushan P, Hanaee J, Aghazadeh Z et al. Novel nanocomposite scaffold based on gelatin/PLGA-PEG-PLGA hydrogels embedded with TGF-β1 for chondrogenic differentiation of human dental pulp stem cells in vitro. Int J Biol Macromol 2022; 201: 270–287.
Raeisdasteh Hokmabad V, Soodabeh D, Ali R, Salehi R. Design and fabrication of porous biodegradable scaffolds: a strategy for tissue engineering. J Biomater Sci Polym Ed 2017; 28: 1797–1825.
Ohlsson E, Galler K M, Widbiller M. A compilation of study models for dental pulp regeneration. Int J Mol Sci 2022; 23: 14361.
Gathani K M, Raghavendra S S. Scaffolds in regenerative endodontics: a review. Dent Res J 2016; 13: 379–386.
Acknowledgements
In this document, we declare the utilisation of artificial intelligence technology for proofreading and refining the English language. The AI tools employed have significantly contributed to improving the text's clarity, coherence, and overall quality.
Funding
This study was supported by the grants PUENTE, GIR, and INDI (2025-2026)from the University CEU Cardenal Herrera, Valencia, Spain, awarded to the PI, Dr. Salvatore Sauro (SS).
Author information
Authors and Affiliations
Contributions
ML-S: Conceptualisation: SS, EB; data curation: WCH, SHC, MC; formal analysis: WCH, MLS, MC; funding acquisition: SS; investigation: WCH, SHC, MC; methodology SS, EB, MLS; project administration SS; resources: SS; supervision: SS, MLS; validation: SS, EB, MLS, visualisation: SS, MC, EB; roles/writing - original draft: WCH, MLS; writing - review & editing: SS, EB. All authors have given their final approval and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
The authors confirm there are no conflicts of interest. Ethical approval and informed consent were not required for this study, which is a systematic review of previously published literature and does not involve new experiments with human participants or animals.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hsueh, WC., Constantinidou, S., Capoferri, M. et al. Hydrogel scaffold for dental pulp regeneration: a systematic review. Br Dent J (2025). https://doi.org/10.1038/s41415-025-9003-x
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41415-025-9003-x