Abstract
Background
Anti-PD-1 antibodies and BRAF/MEK inhibitors are the two main groups of systemic therapy in the treatment of BRAFV600-mutant advanced melanoma. Until now, data are inconclusive on which therapy to use as first-line treatment. The aim of this study was to use propensity score matching to compare first-line anti-PD-1 monotherapy vs. BRAF/MEK inhibitors in advanced BRAFV600-mutant melanoma patients.
Methods
We selected patients diagnosed between 2014 and 2017 with advanced melanoma and a known BRAFV600-mutation treated with first-line BRAF/MEK inhibitors or anti-PD-1 antibodies, registered in the Dutch Melanoma Treatment Registry. Patients were matched based on their propensity scores using the nearest neighbour and the optimal matching method.
Results
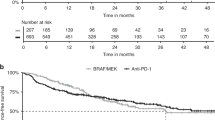
Between 2014 and 2017, a total of 330 and 254 advanced melanoma patients received BRAF/MEK inhibitors and anti-PD-1 monotherapy as first-line systemic therapy. In the matched cohort, patients receiving anti-PD-1 antibodies as a first-line treatment had a higher median and 2-year overall survival compared to patients treated with first-line BRAF/MEK inhibitors, 42.3 months (95% CI: 37.3-NE) vs. 19.8 months (95% CI: 16.7–24.3) and 65.4% (95% CI: 58.1–73.6) vs. 41.7% (95% CI: 34.2–51.0).
Conclusions
Our data suggest that in the matched BRAFV600-mutant advanced melanoma patients, anti-PD-1 monotherapy is the preferred first-line treatment in patients with relatively favourable patient and tumour characteristics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
07 March 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41416-022-01772-z
15 March 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41416-021-01312-1
References
Chapman, P. B., Hauschild, A., Robert, C., Haanen, J. B., Ascierto, P., Larkin, J. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011).
Robert, C., Karaszewska, B., Schachter, J., Rutkowski, P., Mackiewicz, A., Stroiakovski, D. et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 372, 30–39 (2015).
Robert, C., Schachter, J., Long, G. V., Arance, A., Grob, J. J., Mortier, L. et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 372, 2521–2532 (2015).
Schachter, J., Ribas, A., Long, G. V., Arance, A., Grob, J. J., Mortier, L. et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 390, 1853–1862, https://doi.org/10.1016/S0140-6736(17)31601-X (2017).
Larkin, J., Chiarion-Sileni, V., Gonzalez, R., Grob, J. J., Cowey, C. L., Lao, C. D. et al. Combined nivolumab and ipilimumab or monotherapy in untreated Melanoma. N. Engl. J. Med. 373, 23–34 (2015).
NCCN clinical practice guidelines in oncology (NCCN guidelines): cutaneous melanoma. Natl Compr. Cancer Netw. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf (2019).
Pelster, M. S. & Amaria, R. N. Combined targeted therapy and immunotherapy in melanoma: a review of the impact on the tumor microenvironment and outcomes of early clinical trials. Ther. Adv. Med. Oncol. 11, 1–11 (2019).
Schouwenburg, M. G., Suijkerbuijk, K. P. M., Koornstra, R. H. T., Jochems, A., Van Zeijl, M. C. T., Van Den Eertwegh, A. J. M. et al. Switching to immune checkpoint inhibitors upon response to targeted therapy; the road to long- term survival in advanced melanoma patients with highly elevated serum LDH? Cancers (Basel). 11, (2019).
Ugurel, S., Röhmel, J., Ascierto, P. A., Flaherty, K. T., Grob, J. J., Hauschild, A. et al. Survival of patients with advanced metastatic melanoma: the impact of novel therapies–update 2017. Eur. J. Cancer 83, 247–257 (2017).
Rosenbaum, P. R. & Rubin, D. B. The central role of the propensity score in observational studies for causal effects. Matched Sampl. Causal Eff. 70, 170–184 (2006).
Klungel, O. H., Martens, E. P., Psaty, B. M., Grobbee, D. E., Sullivan, S. D., Stricker, B. H. C. et al. Methods to assess intended effects of drug treatment in observational studies are reviewed. J. Clin. Epidemiol. 57, 1223–1231 (2004).
Evaluation, M. & Olmos, A. Propensity scores: a practical introduction using R. J. Multidiscip. Eval. 11, 68–88 (2015).
Jochems, A., Schouwenburg, M. G., Leeneman, B., Franken, M. G., van den Eertwegh, A. J. M., Haanen, J. B. A. G. et al. Dutch Melanoma Treatment Registry: quality assurance in the care of patients with metastatic melanoma in the Netherlands. Eur. J. Cancer 72, 156–165, http://www.ncbi.nlm.nih.gov/pubmed/28030784 (2017).
Manola, J., Atkins, M., Ibrahim, J. & Kirkwood, J. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J. Clin. Oncol. 18, 3782–3793 (2000).
Long, G. V., Grob, J. J., Nathan, P., Ribas, A., Robert, C., Schadendorf, D. et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol. 17, 1743–1754, https://doi.org/10.1016/S1470-2045(16)30578-2 (2016).
Austin, P. C., Grootendorst, P. & Anderson, G. M. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat. Med. 26, 734–753 (2007).
Lin, D. Y. & Wei, L. J. The Robust Inference for the Cox Proportional Hazards Model. J. Am. Stat. Assoc. 84, 1074 (1989).
Austin, P. C. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat. Med. 33, 1242–1258 (2014).
Austin, P. C. A comparison of 12 algorithms for matching on the propensity score. Stat. Med. 33, 1057–1069 (2014).
Austin, P. C. Type I error rates, coverage of confidence intervals, and variance estimation in propensity-score matched analyses. Int. J. Biostat. 5, 1–21 (2009).
Yushak, M., Chapman, P., Robert, C. & Kudchadkar, R. Systemic therapy options for patients with unresectable melanoma. Am. Soc. Clin. Oncol. Educ. B. 37, 661–672 (2017).
Rijksuniversiteit Groningen. Induction Therapy with Vemurafenib and Cobimetinib to Optimize Nivolumab and Ipilimumab Therapy (COWBOY), Identifier: NCT02968303 https://clinicaltrials.gov/ct2/show/study/NCT02968303 (2017).
National Cancer Institute (NCI). DREAMseq (Doublet, Randomized Evaluation in Advanced Melanoma Sequencing) a Phase III Trial, Identifier: NCT02224781. https://clinicaltrials.gov/ct2/show/NCT02224781.
Onlus Fondazione Melanoma. A Three Arms Prospective, Randomized Phase II Study to Evaluate the Best Sequential Approach With Combo Immunotherapy (Ipilimumab/Nivolumab) and Combo Target Therapy (LGX818/MEK162) in Patients With Metastatic Melanoma and BRAF Mutation. Identifier: NCT02. https://clinicaltrials.gov/ct2/show/NCT02631447 (2016).
Robert, C., Long, G. V., Brady, B., Dutriaux, C., Maio, M., Mortier, L. et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372, 320–330 (2015).
Ascierto, P. A., McArthur, G. A., Dréno, B., Atkinson, V., Liszkay, G., Di Giacomo, A. M. et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 17, 1248–1260 (2016).
Luke, J. J., Ghate, S. R., Kish, J., Lee, C. H., McAllister, L., Mehta, S. et al. Targeted agents or immuno-oncology therapies as first-line therapy for BRAF-mutated metastatic melanoma: a real-world study. Future Oncol. 15, 2933–2942 (2019).
Robert, C., Ribas, A., Schachter, J., Arance, A., Grob, J.-J., Mortier, L. et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 20, 1239–1251, https://doi.org/10.1016/S1470-2045(19)30388-2 (2019).
Larkin, J., Chiarion-Sileni, V., Gonzalez, R., Grob, J.-J., Rutkowski, P., Lao, C. D. et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 381, 1535–1546 (2019).
van der Werf, L. R., Voeten, S. C., van Loe, C. M. M., Karthaus, E. G., Wouters, M. W. J. M., Prins, H. A. Data verification of nationwide clinical quality registries. BJS Open 3, 857–864 (2019).
Acknowledgements
We thank all physicians and data managers who registered the patient data in the Dutch Melanoma Treatment Registry.
Author information
Authors and Affiliations
Contributions
J.B. performed the analyses and wrote the manuscript. M.W. was responsible for the study conception and design, analysis and interpretation of data and drafting of the manuscript. D.H. was responsible for the study conception and design, analysis and interpretation of data and drafting of the manuscript. W.B. performed data acquisition, study conception, helped with the analysis and interpretation of the data, drafted the manuscript and performed critical revision and final approval of the manuscript. J.H. performed data acquisition, study conception, helped with the analysis and interpretation of the data, drafted the manuscript and performed critical revision and final approval of the manuscript. C.B. performed data acquisition, study conception, helped with the analysis and interpretation of the data, drafted the manuscript and performed critical revision and final approval of the manuscript. M.A. performed data acquisition, study conception, helped with the analysis and interpretation of the data, drafted the manuscript and performed critical revision and final approval of the manuscript. F.B. performed data acquisition, study conception, helped with the analysis and interpretation of the data, drafted the manuscript, performed critical revision and final approval of the manuscript. J.G. performed data acquisition, study conception, helped with the analysis and interpretation of the data, drafted the manuscript and performed critical revision and final approval of the manuscript. G.H. performed data acquisition, study conception, helped with the analysis and interpretation of the data, drafted the manuscript and performed critical revision and final approval of the manuscript. E.K. performed data acquisition, study conception, helped with the analysis and interpretation of the data, drafted the manuscript and performed critical revision and final approval of the manuscript. D.P. performed data acquisition, study conception, helped with the analysis and interpretation of the data, drafted the manuscript and performed critical revision and final approval of the manuscript. R.R. performed data acquisition, study conception, helped with the analysis and interpretation of the data, drafted the manuscript and performed critical revision and final approval of the manuscript. K.S. performed data acquisition, study conception, helped with the analysis and interpretation of the data, drafted the manuscript and performed critical revision and final approval of the manuscript. A.T. performed data acquisition, study conception, helped with the analysis and interpretation of the data, drafted the manuscript and performed critical revision and final approval of the manuscript. A.V. performed data acquisition, study conception, helped with the analysis and interpretation of the data, drafted the manuscript and performed critical revision and final approval of the manuscript. G.V. performed data acquisition, study conception, helped with the analysis and interpretation of the data, drafted the manuscript and performed critical revision and final approval of the manuscript. M.B. performed data acquisition, study conception, helped with the analysis and interpretation of the data, drafted the manuscript and performed critical revision and final approval of the manuscript. A.E. was responsible for the study conception and design, analysis and interpretation of data and drafting of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
In compliance with Dutch regulations, the DMTR was approved by a medical ethical committee (METC Leiden University Medical Center, 2013) and is not considered subject to the Medical Research Involving Human Subjects Act.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to privacy regulations in the Netherlands but are available from the corresponding author on reasonable request.
Competing interests
A.J.M.V.D.E has consulting/advisory relationships with BMS, Roche, MSD, and Novartis. He received a study grant from Roche. J.W.D.G. has received personal fees outside the submitted work from Bristol-Myers Squibb, Pierre Fabre, Servier, MSD, Novartis. GH consultancy/advisory relationships with Amgen, Bristol-Myers Squibb, Roche, MSD, Pfizer, Novartis and has received research grants not related to this paper from Bristol-Myers Squibb, Seerave. E.K. has consultancy/advisory relationships with Amgen, Bristol-Myers Squibb, Novartis, Merck, Pierre Fabre, and received research grants not related to this paper from Bristol-Myers Squibb. K.P.M.S. has consulting/advisory relationships with BMS and MSD. She received honoraria from Novartis, Pierre Fabre, and Roche. A.V.D.V. has consultancy relationships with Bristol-Myers Squibb, MSD, Roche, Novartis, Pierre Fabre, Pfizer, Sanofi, Ipsen, Eisai. J.H. has advisory relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Celsius Therapeutics, GSK, Immunocore, Ipsen, MSD, Merck Serono, Novartis, Neon Therapeutics, Pfizer, Roche/Genentech, Sanofi, Seattle Genetics and has received research grants not related to this paper from Novartis, Bristol-Myers Squibb, MSD, Neon Therapeutics. C.U.B. has received commercial research grants from Novartis, Bristol-Myers Squibb, and NanoString; is a paid advisory board member for Bristol-Myers Squibb, MSD, Roche, Novartis, GlaxoSmithKline, AstraZeneca, Pfizer, Lilly, GenMab, and Pierre Fabre; and holds ownership interest in Uniti Cars, Neon Therapeutics, and Forty Seven. M.J.B.S. has consultancy relationships with Pierre Fabre, MSD and Novartis. All grants were paid to the institutions. The funders had no role in the writing of this article or decision to submit it for publication. All remaining authors have declared no conflicts of interest
Funding information
For the Dutch Melanoma Treatment Registry (DMTR), the Dutch Institute for Clinical Auditing foundation received a start-up grant from governmental organisation The Netherlands Organization for Health Research and Development (ZonMW, project number 836002002). The DMTR is structurally funded by Bristol-Myers Squibb, Merck Sharpe & Dohme, Novartis and Roche Pharma. Roche Pharma stopped funding in 2019 and Pierre Fabre started funding of the DMTR in 2019. For this work no funding was granted.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The authors have noticed an error in the Abstract.
Supplementary information
Rights and permissions
About this article
Cite this article
van Breeschoten, J., Wouters, M.W.J.M., Hilarius, D.L. et al. First-line BRAF/MEK inhibitors versus anti-PD-1 monotherapy in BRAFV600-mutant advanced melanoma patients: a propensity-matched survival analysis. Br J Cancer 124, 1222–1230 (2021). https://doi.org/10.1038/s41416-020-01229-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-020-01229-1