Abstract
Introduction
Tamoxifen may adversely affect cognitive function by interfering with estrogen action in the brain. Despite growing evidence for a relationship between tamoxifen and cognitive problems, findings remain inconclusive. While some tamoxifen-related side effects seem exposure-dependent with concentrations of tamoxifen or its main metabolite, endoxifen, this has never been investigated for cognitive function. We investigated cognitive function after two years of tamoxifen and its association with tamoxifen and endoxifen exposure.
Methods
135 women with breast cancer completed the Amsterdam Cognition Scan (ACS), an online neuropsychological test battery, after two years of tamoxifen. Test scores were converted to standardized Z-scores based on a matched ‘no-cancer’ control group. Tamoxifen and endoxifen concentrations and tamoxifen dose were regressed separately on cognitive functioning.
Results
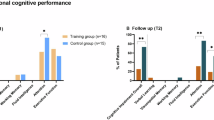
Patients reported mild cognitive complaints and had worse verbal learning, processing speed, executive functioning, and motor functioning compared to matched controls. After correcting for age, mean tamoxifen and endoxifen levels, as well as tamoxifen dose, were associated with worse performance on several cognitive domains.
Conclusion
Tamoxifen is adversely associated with objective as well as self-reported cognitive function, which may depend on the level of exposure to tamoxifen and endoxifen. Further research is warranted to confirm this hypothesis.
Highlights
-
Tamoxifen is associated with worse processing speed, verbal memory, executive function, and motor functioning.
-
The prevalence of cognitive impairment is twice as high in women taking tamoxifen than in controls without cancer.
-
Effects of tamoxifen and its metabolite endoxifen seem to be exposure dependent, especially in younger women.
-
Self-reported cognitive difficulties are associated with tamoxifen-related side effects, anxiety, depression, and fatigue.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Loibl S, André F, Bachelot T, Barrios CH, Bergh J, Burstein HJ, et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up✯. Ann Oncol. 2024;35:159–82.
Land SR, Wickerham DL, Costantino JP, Ritter MW, Vogel VG, Lee M, et al. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2742–51.
Martinkovich S, Shah D, Planey SL, Arnott JA. Selective estrogen receptor modulators: tissue specificity and clinical utility. Clin Inter Aging. 2014;9:1437–52.
Stearns V, Ullmer L, López JF, Smith Y, Isaacs C, Hayes DF. Hot flushes. Lancet. 2002;360:1851–61.
Arevalo MA, Azcoitia I, Garcia-Segura LM. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev. Neurosci. 2015;16:17–29.
Fester L, Prange-Kiel J, Jarry H, Rune GM. Estrogen synthesis in the hippocampus. Cell Tissue Res. 2011;345:285–94.
Irwin RW, Yao J, Hamilton RT, Cadenas E, Brinton RD, Nilsen J. Progesterone and estrogen regulate oxidative metabolism in brain mitochondria. Endocrinology. 2008;149:3167–75.
Sunday L, Osuna C, Krause DN, Duckles SP. Age alters cerebrovascular inflammation and effects of estrogen. Am J Physiol Heart Circ Physiol. 2007;292:H2333–40.
Švob Štrac D, Pivac N, Mück-Šeler D. The serotonergic system and cognitive function. Transl Neurosci. 2016;7:35–49.
Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–84.
Lien EA, Solheim E, Ueland PM. Distribution of tamoxifen and its metabolites in rat and human tissues during steady-state treatment. Cancer Res. 1991;51:4837–44.
Novick AM, Scott AT, Neill Epperson C, Schneck CD. Neuropsychiatric effects of tamoxifen: Challenges and opportunities. Front Neuroendocrinol. 2020;59:100869.
Buwalda B, Schagen SB. Is basic research providing answers if adjuvant anti-estrogen treatment of breast cancer can induce cognitive impairment? Life Sci. 2013;93:581–8.
Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015;65:123–38.
Whittaker AL, George RP, O’Malley L. Prevalence of cognitive impairment following chemotherapy treatment for breast cancer: a systematic review and meta-analysis. Sci. Rep. 2022;12:2135.
Schilder CM, Seynaeve C, Beex LV, Boogerd W, Linn SC, Gundy CM, et al. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J Clin Oncol. 2010;28:1294–1300.
Lee Meeuw Kjoe PR, Kieffer JM, Small BJ, Boogerd W, Schilder CM, van der Wall E, et al. Effects of tamoxifen and exemestane on cognitive function in postmenopausal patients with breast cancer. JNCI Cancer Spectr. 2023;7:pkad022.
Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of hormonal therapy in early stage breast cancer patients: a prospective study. Psycho-Oncol. 2009;18:811–21.
Underwood EA, Rochon PA, Moineddin R, Lee PE, Wu W, Pritchard KI, et al. Cognitive sequelae of endocrine therapy in women treated for breast cancer: a meta-analysis. Breast Cancer Res. Treat. 2018;168:299–310.
Van Dyk K, Crespi CM, Bower JE, Castellon SA, Petersen L, Ganz PA. The cognitive effects of endocrine therapy in survivors of breast cancer: A prospective longitudinal study up to 6 years after treatment. Cancer. 2019;125:681–9.
Mulder TAM, de With M, Del Re M, Danesi R, Mathijssen RHJ, van Schaik RHN. Clinical CYP2D6 Genotyping to Personalize Adjuvant Tamoxifen Treatment in ER-Positive Breast Cancer Patients: Current Status of a Controversy. Cancers. 2021;13:771.
Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–64.
Madlensky L, Natarajan L, Tchu S, Pu M, Mortimer J, Flatt SW, et al. Tamoxifen Metabolite Concentrations, CYP2D6 Genotype, and Breast Cancer Outcomes. Clin Pharm Ther. 2011;89:718–25.
Saladores P, Mürdter T, Eccles D, Chowbay B, Zgheib NK, Winter S, et al. Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharmacogenom J. 2015;15:84–94.
Helland T, Henne N, Bifulco E, Naume B, Borgen E, Kristensen VN, et al. Serum concentrations of active tamoxifen metabolites predict long-term survival in adjuvantly treated breast cancer patients. Breast Cancer Res. 2017;19:125.
Sanchez-Spitman AB, Moes DAR, Swen JJ, Dezentjé VO, Lambrechts D, Neven P, et al. Exposure-response analysis of endoxifen serum concentrations in early-breast cancer. Cancer Chemother Pharm. 2020;85:1141–52.
Braal CL, Beijnen JH, Koolen SLW, Oomen-de Hoop E, Steeghs N, Jager A, et al. Relevance of Endoxifen Concentrations: Absence of Evidence Is Not Evidence of Absence. J Clin Oncol. 2019;37:1980–1.
Braal CL, Jager A, Hoop EO, Westenberg JD, Lommen KMWT, de Bruijn P, et al. Therapeutic Drug Monitoring of Endoxifen for Tamoxifen Precision Dosing: Feasible in Patients with Hormone-Sensitive Breast Cancer. Clin Pharmacokinet. 2022;61:527–37.
Fox P, Balleine RL, Lee C, Gao B, Balakrishnar B, Menzies AM, et al. Dose Escalation of Tamoxifen in Patients with Low Endoxifen Level: Evidence for Therapeutic Drug Monitoring-The TADE Study. Clin Cancer Res. 2016;22:3164–71.
Buijs SM, Oomen-de Hoop E, Braal CL, van Rosmalen MM, Drooger JC, van Rossum-Schornagel QC, et al. The impact of endoxifen-guided tamoxifen dose reductions on endocrine side-effects in patients with primary breast cancer. ESMO Open. 2023;8:100786.
Binkhorst L, Mathijssen RH, Ghobadi Moghaddam-Helmantel IM, de Bruijn P, van Gelder T, Wiemer EAC, et al. Quantification of tamoxifen and three of its phase-I metabolites in human plasma by liquid chromatography/triple-quadrupole mass spectrometry. J Pharm Biomed Anal. 2011;56:1016–23.
Agema BC, Buijs SM, Sassen SDT, Mürdter TE, Schwab M, Koch BCP, et al. Toward model-informed precision dosing for tamoxifen: A population-pharmacokinetic model with a continuous CYP2D6 activity scale. Biomed Pharmacother. 2023;160:114369.
Feenstra HEM, Murre JMJ, Vermeulen IE, Kieffer JM, Schagen SB. Reliability and validity of a self-administered tool for online neuropsychological testing: The Amsterdam Cognition Scan. J Clin Exp Neuropsychol. 2018;40:253–73.
Feenstra HE, Vermeulen IE, Murre JM, Schagen SB. Online Self-Administered Cognitive Testing Using the Amsterdam Cognition Scan: Establishing Psychometric Properties and Normative Data. J Med Internet Res. 2018;20:e192.
Jones D, Vichaya EG, Wang XS, Williams LA, Shah ND, Thomas SK, et al. Validation of the M. D. Anderson Symptom Inventory multiple myeloma module. J Hematol Oncol. 2013;6:13.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70.
Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–25.
Luijendijk M, Lee Meeuw Kjoe P, Wortelboer N, Van Ommen - Nijhof A, Agelink van Rentergem J, Vermeulen I, et al. Cognitive function in patients with HR+ advanced breast cancer treated with endocrine therapy with or without CDK4/6 inhibitors in the SONIA trial. J Clin Oncol. 2024;42:1016.
Fallowfield LJ, Leaity SK, Howell A, Benson S, Cella D. Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat. 1999;55:187–97.
Lezak MD. Neuropsychological assessment (Oxford University Press: USA, 2004).
Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12:703–8.
Ingraham L, Aiken C. An Empirical Approach to Determining Criteria for Abnormality in Test Batteries With Multiple Measures. Neuropsychology. 1996;10:120–4.
Salthouse TA. Selective review of cognitive aging. J Int Neuropsychol Soc. 2010;16:754–60.
Souwer ETD, Sanchez-Spitman A, Moes DJAR, Gelderblom H, Swen JJ, Portielje JEA, et al. Tamoxifen pharmacokinetics and pharmacodynamics in older patients with non-metastatic breast cancer. Breast Cancer Res Treat. 2023;199:471–8.
Lien EA, Søiland H, Lundgren S, Aas T, Steen VM, Mellgren G, et al. Serum concentrations of tamoxifen and its metabolites increase with age during steady-state treatment. Breast Cancer Res. Treat. 2013;141:243–8.
Mueller-Schoell A, Klopp-Schulze L, Schroth W, Mürdter T, Michelet R, Brauch H, et al. Obesity Alters Endoxifen Plasma Levels in Young Breast Cancer Patients: A Pharmacometric Simulation Approach. Clin Pharm Ther. 2020;108:661–70.
Ohsumi S, Shimozuma K, Ohashi Y, Shinji M, Hozumi Y, Mukai H, et al. Health-related quality of life and psychological distress of breast cancer patients after surgery during a phase III randomized trial comparing continuation of tamoxifen with switching to anastrozole after adjuvant tamoxifen for 1–4 years: N-SAS BC 03. Breast Cancer Res Treat. 2011;127:143–52.
Takei H, Ohsumi S, Shimozuma K, Takehara M, Suemasu K, Ohashi Y, et al. Health-related quality of life, psychological distress, and adverse events in postmenopausal women with breast cancer who receive tamoxifen, exemestane, or anastrozole as adjuvant endocrine therapy: National Surgical Adjuvant Study of Breast Cancer 04 (N-SAS BC 04). Breast Cancer Res Treat. 2012;133:227–36.
Cella D, Fallowfield L, Barker P, Cuzick J, Locker G, Howell A, et al. Quality of Life of Postmenopausal Women in the ATAC (“Arimidex”, Tamoxifen, Alone or in Combination) Trial after Completion of 5 years’ Adjuvant Treatment for Early Breast Cancer. Breast Cancer Res Treat. 2006;100:273–84.
Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010;28:4434–40.
Luine VN. Estradiol and cognitive function: Past, present and future. Horm Behav. 2014;66:602–18.
Zwart W, Terra H, Linn SC, Schagen SB. Cognitive effects of endocrine therapy for breast cancer: keep calm and carry on? Nat Rev Clin Oncol. 2015;12:597–606.
Boyle CP, Raji CA, Erickson KI, Lopez OL, Becker JT, Gach HM, et al. Estrogen, brain structure, and cognition in postmenopausal women. Hum Brain Mapp. 2021;42:24–35.
Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum Brain Mapp. 2014;35:847–65.
Dieni CV, Contemori S, Biscarini A, Panichi R. De Novo Synthesized Estradiol: A Role in Modulating the Cerebellar Function. Int J Mol Sci. 2020;21:3316.
Li M, Caeyenberghs K. Longitudinal assessment of chemotherapy-induced changes in brain and cognitive functioning: A systematic review. Neurosci Biobehav Rev. 2018;92:304–17.
Chen X, He X, Tao L, Li J, Wu J, Zhu C, et al. The Working Memory and Dorsolateral Prefrontal-Hippocampal Functional Connectivity Changes in Long-Term Survival Breast Cancer Patients Treated with Tamoxifen. Int J Neuropsychopharmacol. 2017;20:374–82.
McDonald BC, Van Dyk K, Deardorff RL, Bailey JN, Zhai W, Carroll JE, et al. Multimodal MRI examination of structural and functional brain changes in older women with breast cancer in the first year of antiestrogen hormonal therapy. Breast Cancer Res. Treat. 2022;194:113–26.
Kjoe P, van der Wall E, Schagen SB. Endocrine Therapy With or Without CDK4/6 Inhibitors in Women With Hormone-receptor Positive Breast Cancer: What do we Know About the Effects on Cognition? Clin Breast Cancer. 2022;22:191–9.
Hutchinson AD, Hosking JR, Kichenadasse G, Mattiske JK, Wilson C. Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review. Cancer Treat Rev. 2012;38:926–34.
Baltussen JC, Derks MGM, Lemij AA, de Glas NA, Fiocco M, Linthorst-Niers EMH, et al. Association between endocrine therapy and cognitive decline in older women with early breast cancer: Findings from the prospective CLIMB study. Eur J Cancer. 2023;185:1–10.
Funding
The TOTAM-study was supported by an unrestricted MRACE grant (Erasmus MC, The Netherlands) [grant number 2017-108].
Author information
Authors and Affiliations
Contributions
ML, SB, AJ, SK, EW, SS and RM designed the research. ML, SB and RM acquired data. ML, SB and SS analysed the data. ML and SB wrote the manuscript. All authors revised the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of the Erasmus Medical Center (MEC 2017-548) and was performed according the Declaration of Helsinki. All patients provided written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luijendijk, M.J., Buijs, S.M., Jager, A. et al. Effects of tamoxifen on cognitive function in patients with primary breast cancer. Br J Cancer 132, 180–187 (2025). https://doi.org/10.1038/s41416-024-02914-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-024-02914-1