Abstract
Background
Cutaneous neurofibroma (cNF) presenting with pain and itch substantially affects the quality of life. The CXCL10/CXCR3 axis, a well-known chemokine signaling pathway involved in pain and itch transmission, has recently been implicated in neurofibroma development. Our study aims to investigate the expression patterns and potential roles of the CXCL10/CXCR3 axis in pain and itch associated with cNFs.
Methods
We examined the expression of CXCL10/CXCR3 and immune cell profiles in 53 human solitary cNFs through immunohistochemical staining. The Chinese version of the Short-form McGill Pain Questionnaire and the Chinese Eppendorf Itch Questionnaire were used to assess pain and itch symptoms of cNF tumors, respectively.
Results
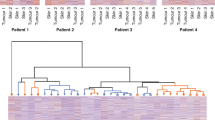
Elevated expression of CXCL10/CXCR3 was observed in tumoral and dermal parts of symptomatic cNFs. The percentage of mast cells expressing CXCL10, but not CXCR3, was significantly higher in symptomatic cNFs compared to asymptomatic cNFs (51.18% vs. 19.07%, respectively, p < 0.0001). The symptomatic cNFs exhibited significantly higher intraepidermal nerve fiber density compared to asymptomatic cNFs (p = 0.009).
Conclusions
Our study suggests that CXCL10, potentially mediated by mast cells, may contribute to sensory dysfunction in cNF and may be a target for treating the pain and itch symptoms associated with cNFs.

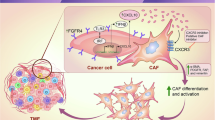
Our study suggests a model in which the CXCL10/CXCR3 pathway plays a role in inducing pain and itch in cNFs, potentially through mast cell mediation. Mast cells may increase the secretion of CXCL10, thereby contributing to pain and itch in cNF, making them a potential target for treating these symptoms. Created in BioRender. Pham, Q. (2025) https://BioRender.com/i89y356.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated in this study are accessible from the corresponding author upon reasonable inquiry.
References
Snijders RAH, Brom L, Theunissen M, van den Beuken-van Everdingen MHJ. Update on prevalence of pain in patients with cancer 2022: a systematic literature review and meta-analysis. Cancers (Basel). 2023;15:591.
Kılıç A, Gül Ü, Soylu S. Skin findings in internal malignant diseases. Int J Dermatol. 2007;46:1055–60.
Weisshaar E, Dalgard F. Epidemiology of itch: adding to the burden of skin morbidity. Acta Derm Venereol. 2009;89:339–50.
Van Den Beuken-Van MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. 2016;51:1070–90.
Joshy G, Khalatbari-Soltani S, Soga K, Butow P, Laidsaar-Powell R, Koczwara B, et al. Pain and its interference with daily living in relation to cancer: a comparative population-based study of 16,053 cancer survivors and 106,345 people without cancer. BMC Cancer. 2023;23:774.
Larson VA, Tang O, Ständer S, Kang S, Kwatra SG. Association between itch and cancer in 16,925 patients with pruritus: Experience at a tertiary care center. J Am Acad Dermatol. 2019;80:931–7.
Granström S, Langenbruch A, Augustin M, Mautner VF. Psychological burden in adult neurofibromatosis type 1 patients: impact of disease visibility on body image. Dermatology. 2012;224:160–7.
Guiraud M, Bouroubi A, Beauchamp R, Bocquet A, Grégoire JM, Rauly-Lestienne I, et al. Cutaneous neurofibromas: patients’ medical burden, current management and therapeutic expectations: results from an online European patient community survey. Orphanet J Rare Dis. 2019;14:286.
Buono FD, Grau LE, Sprong ME, Morford KL, Johnson KJ, Gutmann DH. Pain symptomology, functional impact, and treatment of people with Neurofibromatosis type 1. J Pain Res. 2019;12:2555–61.
Wolters PL, Burns KM, Martin S, Baldwin A, Dombi E, Toledo‐Tamula MA, et al. Pain interference in youth with neurofibromatosis type 1 and plexiform neurofibromas and relation to disease severity, social‐emotional functioning, and quality of life. Am J Med Genet A. 2015;167:2103–13.
Ferner RE, Thomas M, Mercer G, Williams V, Leschziner GD, Afridi SK, et al. Evaluation of quality of life in adults with neurofibromatosis 1 (NF1) using the Impact of NF1 on Quality Of Life (INF1-QOL) questionnaire. Health Qual Life Outcomes. 2017;15:1–6.
Ly I, Romo CG, Gottesman S, Kelly KM, Kornacki D, York Z, et al. Target product profile for cutaneous neurofibromas: clinical trials to prevent, arrest, or regress cutaneous neurofibromas. J Invest Dermatol. 2023;143:1388–96.
Brosseau JP, Pichard DC, Legius EH, Wolkenstein P, Lavker RM, Blakeley JO, et al. The biology of cutaneous neurofibromas: consensus recommendations for setting research priorities. Neurology. 2018;91:S14–S20.
Prada CE, Jousma E, Rizvi TA, Wu J, Dunn RS, Mayes DA, et al. Neurofibroma-associated macrophages play roles in tumor growth and response to pharmacological inhibition. Acta Neuropathol. 2013;125:159–68.
Riccardi V. Current utilization of mast cell stabilizers for preemptive treatment of NF1 neurofibromas. Neuro Open J. 2015;2:67–73.
Kallionpää RA, Ahramo K, Martikkala E, Fazeli E, Haapaniemi P, Rokka A, et al. Mast cells in human cutaneous neurofibromas: density, subtypes, and association with clinical features in neurofibromatosis 1. Dermatology. 2022;238:329–39.
Rice FL, Houk G, Wymer JP, Gosline SJC, Guinney J, Wu J, et al. The evolution and multi-molecular properties of NF1 cutaneous neurofibromas originating from C-fiber sensory endings and terminal Schwann cells at normal sites of sensory terminations in the skin. PLoS One. 2019;14:e0216527.
Jouhilahti EM, Peltonen S, Callens T, Jokinen E, Heape AM, Messiaen L, et al. The development of cutaneous neurofibromas. Am J Pathol. 2011;178:500–5.
Radomska KJ, Coulpier F, Gresset A, Schmitt A, Debbiche A, Lemoine S, et al. Cellular origin, tumor progression, and pathogenic mechanisms of cutaneous neurofibromas revealed by mice with Nf1 knockout in boundary cap cells. Cancer Discov. 2019;9:130–47.
Fletcher JS, Wu J, Jessen WJ, Pundavela J, Miller JA, Dombi E, et al. Cxcr3-expressing leukocytes are necessary for neurofibroma formation in mice. JCI Insight. 2019;4:e98601.
Meixiong J, Anderson M, Limjunyawong N, Sabbagh MF, Hu E, Mack MR, et al. Activation of mast-cell-expressed Mas-related G protein-coupled receptors drives non-histaminergic itch. Immunity. 2019;50:1163–71.
Hua X, Ge S, Zhang M, Mo F, Zhang L, Zhang J, et al. Pathogenic roles of CXCL10 in experimental autoimmune prostatitis by modulating macrophage chemotaxis and cytokine secretion. Front Immunol. 2021;12:706027.
Hartl D, Krauss-Etschmann S, Koller B, Hordijk PL, Kuijpers TW, Hoffmann F, et al. Infiltrated neutrophils acquire novel chemokine receptor expression and chemokine responsiveness in chronic inflammatory lung diseases. J Immunol. 2008;181:8053–67.
Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res. 2011;317:620–31.
Kallionpää RA, Peltonen S, Le KM, Martikkala E, Jääskeläinen M, Fazeli E, et al. Characterization of immune cell populations of cutaneous neurofibromas in neurofibromatosis 1. Lab Invest. 2024;104:100285.
Quirk B, Olasz E, Kumar S, Basel D, Whelan H. Photodynamic therapy for benign cutaneous neurofibromas using aminolevulinic acid topical application and 633 nm red light illumination. Photobiomodul Photomed Laser Surg. 2021;9:411–7.
Qu L, Fu K, Yang J, Shimada SG, LaMotte RH. CXCR3 chemokine receptor signaling mediates itch in experimental allergic contact dermatitis. Pain. 2015;156:1737–46.
Walsh CM, Hill RZ, Schwendinger-Schreck J, Deguine J, Brock EC, Kucirek N, et al. Neutrophils promote CXCR3-dependent itch in the development of atopic dermatitis. Elife. 2019;8:e48448.
Li K, Tan YH, Feng SY, Fu KY. CXCR3 signalling partially contributes to the pathogenesis of neuropathic pain in male rodents. J Oral Rehabil. 2022;49:186–94.
Jing PB, Cao DL, Li SS, Zhu M, Bai XQ, Wu XB, et al. Chemokine receptor CXCR3 in the spinal cord contributes to chronic itch in mice. Neurosci Bull. 2018;34:54–63.
Pawlik K, Ciechanowska A, Ciapała K, Rojewska E, Makuch W, Mika J. Blockade of CC chemokine receptor type 3 diminishes pain and enhances opioid analgesic potency in a model of neuropathic pain. Front Immunol. 2021;12:781310.
Bu H, Shu B, Gao F, Liu C, Guan X, Ke C, et al. Spinal IFN-γ-induced protein-10 (CXCL10) mediates metastatic breast cancer-induced bone pain by activation of microglia in rat models. Breast Cancer Res Treat. 2014;143:255–63.
Hirth M, Gandla J, Höper C, Gaida MM, Agarwal N, Simonetti M, et al. CXCL10 and CCL21 promote migration of pancreatic cancer cells toward sensory neurons and neural remodeling in tumors in mice, associated with pain in patients. Gastroenterology. 2020;159:665–81.
Guan XH, Fu QC, Shi D, Bu HL, Song ZP, Xiong BR, et al. Activation of spinal chemokine receptor CXCR3 mediates bone cancer pain through an Akt-ERK crosstalk pathway in rats. Exp Neurol. 2015;263:39–49.
Lee YB, Lee JI, Park HJ, Cho BK. Solitary neurofibromas: does an uncommon site exist? Ann Dermatol. 2012;24:101–2.
Weng HJ, Shih MH, Tsai TF, Song YC, Pan YC, Hu JY, et al. Clinical validation and utility of Chinese Eppendorf Itch Questionnaire in adults with chronic pruritus symptoms. J Formos Med Assoc. 2021;120:492–500.
Darsow U, Mautner V, Bromm B, Scharein E, Ring J. The Eppendorf pruritus questionnaire. Hautarzt. 1997;48:730–3.
Wang JL, Zhang WJ, Gao M, Zhang S, Tian DH, Chen J. A cross-cultural adaptation and validation of the short-form McGill Pain Questionnaire-2: Chinese version in patients with chronic visceral pain. J Pain Res. 2017;10:121–8.
Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–7.
Pan CL, Tseng TJ, Lin YH, Chiang MC, Lin WM, Hsieh ST. Cutaneous innervation in Guillain–Barré syndrome: pathology and clinical correlations. Brain. 2003;126:386–97.
Liao CP, Booker RC, Brosseau JP, Chen Z, Mo J, Tchegnon E, et al. Contributions of inflammation and tumor microenvironment to neurofibroma tumorigenesis. J Clin Invest. 2018;128:2848–61.
Aloyouny AY, Bepari A, Rahman I. Evaluating the role of CXCR3 in pain modulation: a literature review. J Pain Res. 2020;13:1987–2001.
Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, et al. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–9.
Wang WN, Koguchi-Yoshioka H, Nimura K, Watanabe R, Tanemura A, Fujimoto M, et al. Distinct transcriptional profiles in the different phenotypes of neurofibroma from the same neurofibromatosis 1 subject. J Invest Dermatol. 2024;144:133–41.
Thomas S, Enders J, Kaiser A, Rovenstine L, Heslop L, Hauser W, et al. Abnormal intraepidermal nerve fiber density in disease: A scoping review. Front Neurol. 2023;14:1161077.
Meixiong J, Dong X, Weng HJ. Neuropathic itch. Cells. 2020;9:2263.
Pereira MP, Wiegmann H, Agelopoulos K, Ständer S. Neuropathic itch: routes to clinical diagnosis. Front Med (Lausanne). 2021;8:641746.
Chen O, He Q, Han Q, Furutani K, Gu Y, Olexa M, et al. Mechanisms and treatments of neuropathic itch in a mouse model of lymphoma. J Clin Invest. 2023;133:e160807.
Gupta K, Harvima IT. Mast cell‐neural interactions contribute to pain and itch. Immunol Rev. 2018;282:168–87.
Acknowledgements
This work was supported by the Higher Education Sprout Project (DP2-110-21121-01-N-12-02, DP2-111-21121-01-N-01-02) by the Ministry of Education (MOE) in Taiwan, by Taipei Medical University-Shuang Ho Hospital, Ministry of Health and Welfare (111YSR-06), and by National Science and Technology Council in Taiwan (110-2314-B-038 -028 -MY3).
Author information
Authors and Affiliations
Contributions
HJW and TTQP designed the study and performed experiments, analysed the data, wrote the manuscript, and prepared the figures. CPL, WRL, KHL, TSY, YHJS, YHL and HJW supervised the study, discussed, and contributed to the manuscript. HJW, WRL, CLC, YHS, YWC, DL, HCW, and BJC recruited the patients and processed the human specimens. All authors reviewed and approved the final revised version.
Corresponding author
Ethics declarations
Competing interests
The authors declared no competing interests.
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of Taipei Medical University (TMU-REC No. N202103158). Patients were recruited at the Department of Dermatology, Taipei Medical University-Shuang Ho Hospital. The recruitment of patients and collection of tumor specimens adhered to the principles of the Declaration of Helsinki. All participants provided written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pham, T.T.Q., Liao, CP., Shih, YH. et al. Enhanced CXCL10 expression in mast cells for cutaneous neurofibroma presenting with pain and itch. Br J Cancer 132, 611–621 (2025). https://doi.org/10.1038/s41416-025-02956-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-02956-z