Abstract
Background
Colorectal cancer liver metastasis (CRLM) remains a major challenge in oncology, with the tumor microenvironment playing a crucial role in disease progression. This study investigates the function of the Tripartite Motif Containing 26 (TRIM26) in the CRLM microenvironment, focusing on its regulation of tumor-associated macrophage (TAM) polarization and its implications for metastatic growth.
Methods
Using established mouse CRLM models, we characterized TAM phenotypes using flow cytometry and immunohistochemistry. In vitro co-culture experiments evaluated the effects of Trim26-deficient bone marrow-derived macrophages (BMDMs) on tumor cell behavior. Western blotting and luciferase reporter assays were employed to elucidate the underlying molecular mechanisms.
Results
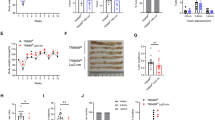
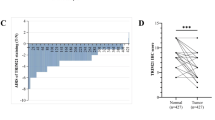
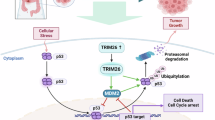
Trim26 knockout mice exhibited significantly reduced liver metastasis and an increased proportion of M1-like TAMs. Trim26-deficient BMDMs suppressed tumor cell migration and proliferation. TRIM26 modulates macrophage polarization by inhibiting the NF-κB signaling pathway. Specifically, TRIM26 interacts with TRAF2 through its PRY domain and inhibits the K63-linked ubiquitination of TRAF2, thereby attenuating NF-κB pathway activation. Furthermore, clinical CRLM samples revealed a negative correlation between TRIM26 expression and M1-like TAM infiltration.
Conclusion
We identified TRIM26 as a potential therapeutic target for CRLM, providing novel insights into tumor-stromal microenvironment interactions and offering new strategies to improve patient outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data generated in this study are available within the article and its supplementary information files.
References
Adam R, De Gramont A, Figueras J, Guthrie A, Kokudo N, Kunstlinger F, et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012;17:1225–39.
Zheng X, Turkowski K, Mora J, Brne B, Seeger W, Weigert A, et al. Redirecting tumor-associated macrophages to become tumoricidal effectors as a novel strategy for cancer therapy. Oncotarget. 2017;8:48436–52.
Douaiher J, Ravipati A, Grams B, Chowdhury S, Alatise O, Are C. Colorectal cancer-global burden, trends, and geographical variations. J Surg Oncol. 2017;115:619–30.
Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–64.
van der Pool AE, Damhuis RA, Ijzermans JN, de Wilt JH, Eggermont AM, Kranse R, et al. Trends in incidence, treatment and survival of patients with stage IV colorectal cancer: a population-based series. Colorectal Dis. 2012;14:56–61.
Ito K, Govindarajan A, Ito H, Fong Y. Surgical treatment of hepatic colorectal metastasis: evolving role in the setting of improving systemic therapies and ablative treatments in the 21st century. Cancer J. 2010;16:103–10.
Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346–55.
Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chien CRC, Makhson A, et al. Cetuximab and Chemotherapy as Initial Treatment for Metastatic Colorectal Cancer. N. Engl J Med. 2009;360:1408–17.
Takasu C, Yamashita S, Morine Y, Yoshikawa K, Tokunaga T, Nishi M, et al. The role of the immunoescape in colorectal cancer liver metastasis. PLoS One. 2021;16:e0259940.
Ngambenjawong C, Gustafson HH, Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev. 2017;114:206–21.
Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61.
Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood. 2006;107:2112–22.
Kashfi K, Kannikal J, Nath N. Macrophage Reprogramming and Cancer Therapeutics: Role of iNOS-Derived NO. Cells-Basel. 2021;10:3194.
Li M, Lai X, Zhao Y, Zhang Y, Li M, Li D, et al. Loss of NDRG2 in liver microenvironment inhibits cancer liver metastasis by regulating tumor associate macrophages polarization. Cell Death Dis. 2018;9:248.
Pan Y, Yu Y, Wang X, Zhang T. Tumor-Associated Macrophages in Tumor Immunity. Front Immunol. 2020;11:583084.
Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, et al. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol. 2016;18:549–60.
Zhou PT, Li QQ, Su SW, Dong WH, Zong SY, Ma Q, et al. Interleukin 37 Suppresses M1 Macrophage Polarization Through Inhibition of the Notch1 and Nuclear Factor Kappa B Pathways. Front Cell Dev Biol. 2020;8:56.
Fan H, Wu Q, Peng LP, Li D, Dong YD, Cao M, et al. Phyllolobium chinense Fisch Flavonoids (PCFF) Suppresses the M1 Polarization of LPS-Stimulated RAW264.7 Macrophages by Inhibiting NF-kappa B/iNOS Signaling Pathway. Front Pharmacol. 2020;11:864.
Hagemann T, Biswas SK, Lawrence T, Sica A, Lewis CE. Regulation of macrophage function in tumors: the multifaceted role of NF-kappaB. Blood. 2009;113:3139–46.
Wang Q, Jiang H, Li Y, Chen W, Li H, Peng K, et al. Targeting NF-kB signaling with polymeric hybrid micelles that co-deliver siRNA and dexamethasone for arthritis therapy. Biomaterials. 2017;122:10–22.
Chung JY, Park YC, Ye H, Wu H. All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J Cell Sci. 2002;115:679–88.
Bradley JR, Pober JS. Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene. 2001;20:6482–91.
So T. The immunological significance of tumor necrosis factor receptor-associated factors (TRAFs). Int Immunol. 2022;34:7–20.
Tao JL, Luo M, Sun H, Zhao HM, Sun QS, Huang ZM. Overexpression of tripartite motif containing 26 inhibits non-small cell lung cancer cell growth by suppressing PI3K/AKT signaling. Kaohsiung J Med Sci. 2020;36:417–22.
Hatakeyama S. TRIM Family Proteins: Roles in Autophagy, Immunity, and Carcinogenesis. Trends Biochem Sci. 2017;42:297–311.
Wang Y, He H, Yang L, Wen B, Dai JF, Zhang Q, et al. TRIM26 functions as a novel tumor suppressor of hepatocellular carcinoma and its downregulation contributes to worse prognosis. Biochem Biophys Res Co. 2015;463:458–65.
Lyu XM, Zhu XW, Zhao ML, Zuo XB, Huang ZX, Liu X, et al. A regulatory mutant on TRIM26 conferring the risk of nasopharyngeal carcinoma by inducing low immune response. Cancer Med-Us. 2018;7:3848–61.
Wang KF, Chai LY, Qiu ZG, Zhang YD, Gao HY, Zhang XZ. Overexpression of TRIM26 suppresses the proliferation, metastasis, and glycolysis in papillary thyroid carcinoma cells. J Cell Physiol. 2019;234:19019–27.
Zheng D, Ning J, Deng H, Ruan Y, Cheng F. TRIM26 inhibits clear cell renal cell carcinoma progression through destabilizing ETK and thus inactivation of AKT/mTOR signaling. J Transl Med. 2024;22:481.
Sun Y, Lin P, Zhou X, Ren Y, He Y, Liang J, et al. TRIM26 promotes non-small cell lung cancer survival by inducing PBX1 degradation. Int J Biol Sci. 2023;19:2803–16.
Li X, Yuan J, Song C, Lei Y, Xu J, Zhang G, et al. Deubiquitinase USP39 and E3 ligase TRIM26 balance the level of ZEB1 ubiquitination and thereby determine the progression of hepatocellular carcinoma. Cell Death Differ. 2021;28:2315–32.
Wang W, Lei Y, Zhang G, Li X, Yuan J, Li T, et al. USP39 stabilizes β-catenin by deubiquitination and suppressing E3 ligase TRIM26 pre-mRNA maturation to promote HCC progression. Cell death Dis. 2023;14:63.
Li T, Zhong W, Li M, Shao Z, Zhang G, Wang W, et al. TRIM26 deficiency enhancing liver regeneration through macrophage polarization and β-catenin pathway activation. Cell Death Dis. 2024;15:453.
Wang H, Lou J, Liu H, Liu Y, Xie B, Zhang W, et al. TRIM59 deficiency promotes M1 macrophage activation and inhibits colorectal cancer through the STAT1 signaling pathway. Sci Rep. 2024;14:16081.
Kitamura T, Fujishita T, Loetscher P, Revesz L, Hashida H, Kizaka-Kondoh S, et al. Inactivation of chemokine (C-C motif) receptor 1 (CCR1) suppresses colon cancer liver metastasis by blocking accumulation of immature myeloid cells in a mouse model. Proc Natl Acad Sci USA. 2010;107:13063–8.
Bleriot C, Li S, Kairi M, Newell E, Ginhoux F. Kupffer Cell Characterization by Mass Cytometry. Methods Mol Biol. 2020;2164:87–99.
Daemen S, Chan MM, Schilling JD. Comprehensive analysis of liver macrophage composition by flow cytometry and immunofluorescence in murine NASH. STAR Protoc. 2021;2:100511.
He R, Li Y, Han C, Lin R, Qian W, Hou X. L-Fucose ameliorates DSS-induced acute colitis via inhibiting macrophage M1 polarization and inhibiting NLRP3 inflammasome and NF-kB activation. Int Immunopharmacol. 2019;73:379–88.
Zhang L, Previn R, Lu L, Liao RF, Jin Y, Wang RK. Crocin, a natural product attenuates lipopolysaccharide-induced anxiety and depressive-like behaviors through suppressing NF-kB and NLRP3 signaling pathway. Brain Res Bull. 2018;142:352–9.
Nasirzade J, Kargarpour Z, Hasannia S, Strauss FJ, Gruber R. Platelet-rich fibrin elicits an anti-inflammatory response in macrophages in vitro. J Periodontol. 2020;91:244–52.
Chen S, Ye J, Chen X, Shi J, Wu W, Lin W, et al. Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-kappaB pathway dependent of HDAC3. J Neuroinflammation. 2018;15:150.
Bessa-Goncalves M, Silva AM, Bras JP, Helmholz H, Luthringer-Feyerabend BJC, Willumeit-Romer R, et al. Fibrinogen and magnesium combination biomaterials modulate macrophage phenotype, NF-kB signaling and crosstalk with mesenchymal stem/stromal cells. Acta Biomater. 2020;114:471–84.
Lu C-H, Yeh D-W, Lai C-Y, Liu Y-L, Huang L-R, Lee AY-L, et al. USP17 mediates macrophage-promoted inflammation and stemness in lung cancer cells by regulating TRAF2/TRAF3 complex formation. Oncogene. 2018;37:6327–40.
Dupoux A, Cartier J, Cathelin S, Filomenko R, Solary E, Dubrez-Daloz L. cIAP1-dependent TRAF2 degradation regulates the differentiation of monocytes into macrophages and their response to CD40 ligand. Blood. 2009;113:175–85.
Liu C, Yao ZY, Wang JN, Zhang W, Yang Y, Zhang Y, et al. Macrophage-derived CCL5 facilitates immune escape of colorectal cancer cells via the p65/STAT3-CSN5-PD-L1 pathway (vol 74, pg 2351, 2019). Cell Death Differ. 2020;27:2293–2293.
Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer. 2010;10:112.
Zheng C, Kabaleeswaran V, Wang Y, Cheng G, Wu H. Crystal Structures of the TRAF2: cIAP2 and the TRAF1: TRAF2: cIAP2 Complexes: Affinity, Specificity, and Regulation. Mol Cell. 2010;38:101–13.
Vandenabeele P, Bertrand MJM. The role of the IAP E3 ubiquitin ligases in regulating pattern-recognition receptor signalling. Nat Rev Immunol. 2012;12:833–44.
Yang XD, Sun SC. Targeting signaling factors for degradation, an emerging mechanism for TRAF functions. Immunological Rev. 2015;266:56–71.
Acknowledgements
We thank the Department of Pathology, the 909th hospital, School of Medicine, Xiamen University for providing tissue sections.
Funding
The work was supported by the National Natural Science Foundation of China (82372809 and 81872045), and the Special Fund for Public Welfare Research Institutes of Fujian Province (2023R1001003).
Author information
Authors and Affiliations
Contributions
Conceptualization: WZ, YZ1 (Yuqi Zhang), GS. Formal analysis: WZ, YZ1, WW, ZS, ZX, YZ2 (Yayu Zhang), HC. Funding acquisition: GS. Investigation: WZ, YZ1, ZS, ZX, ZG, DZ, ZZ. Methodology: WZ, YZ1, WW, ZG, GZ, ZZ. Visualization: WZ, YZ1, ZS, ZX. Writing—original draft: WZ, YZ1. Writing—review & editing: WZ, GS.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods in this study were performed in accordance with the relevant guidelines and regulations. Human tissue acquisition and subsequent use were approved by the Medical Ethics Committee of The 909th Hospital, School of Medicine, Xiamen University, and informed consent was obtained from patients/family members. The animal study protocol was approved by the Institutional Animal Care and Use Committee of Xiamen University (IACUC No. XMULAC20180056).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhong, W., Zhang, Y., Wang, W. et al. TRIM26 deficiency potentially suppresses colorectal cancer liver metastasis through NF-κB-mediated M1-like tumor-associated macrophage polarization. Br J Cancer 133, 435–447 (2025). https://doi.org/10.1038/s41416-025-03072-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-03072-8
This article is cited by
-
TRIM26 deficiency promotes liver fibrosis progression by mediating macrophage polarization via the EZH2-STAT1 axis
Hepatology International (2026)