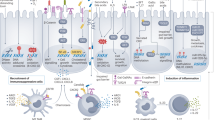
Abstract
Background
Elevating major histocompatibility complex class I (MHC-I) levels in tumour cells can boost antitumour immunity and enhance immunotherapy for colorectal cancer (CRC). Screening an FDA-approved drug library showed that MEK inhibitors (MEKis) significantly increase MHC-I expression in CRC cells, though the mechanisms and antitumour effects of MEKis, as well as their impact on gut microbiota, remain unclear.
Methods
Dual-luciferase reporter system was employed to screen MHC-I inducers. MHC-I expression was analysed using qRT-PCR, flow cytometry, and western blot. OT-I TCR transgenic mice, subcutaneous mouse tumour models, RNA-seq, and ChIP-qPCR were used to identify the underlying mechanism. Gut microbiota was depleted using antibiotics cocktail and analysed via Shotgun sequencing, 16S rRNA sequencing and nontargeted metabolomic sequencing.
Results
MEKis, particularly cobimetinib, increased MHC-I expression by inhibiting PRMT5-mediated repression of NLRC5, boosting CD8+ T cell-mediated immunity and enhancing PD-L1 blockade efficacy. Cobimetinib also altered gut microbiota, reducing L-arginine via arginase production, which compromised antitumour immunity. Arginase inhibition or L-arginine supplementation restored immune responses.
Conclusions
This study uncovers a novel mechanism of MEKi-induced MHC-I expression and highlights the interplay between gut microbiota and antitumour immunity, providing insights for MEKi-based CRC immunotherapy.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Messersmith WA. Nccn guidelines updates: management of metastatic colorectal cancer. J Natl Compr Cancer Netw. 2019;17:599–601.
Zaanan A, Shi Q, Taieb J, Alberts SR, Meyers JP, Smyrk TC, et al. Role of deficient DNA mismatch repair status in patients with stage iii colon cancer treated with folfox adjuvant chemotherapy: a pooled analysis from 2 randomized clinical trials. JAMA Oncol. 2018;4:379–83.
Jhunjhunwala S, Hammer C, Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer. 2021;21:298–312.
Dendrou CA, Petersen J, Rossjohn J, Fugger L. Hla variation and disease. Nat Rev Immunol. 2018;18:325–39.
Burr ML, Sparbier CE, Chan KL, Chan YC, Kersbergen A, Lam EYN, et al. An evolutionarily conserved function of polycomb silences the MHC class I antigen presentation pathway and enables immune evasion in cancer. Cancer Cell. 2019;36:385–401.e8.
Stachura P, Liu W, Xu HC, Wlodarczyk A, Stencel O, Pandey P, et al. Unleashing T cell anti-tumor immunity: new potential for 5-nonloxytryptamine as an agent mediating MHC-I upregulation in tumors. Mol Cancer. 2023;22:136.
Franklin DA, James JL, Axelrod ML, Balko JM. MEK inhibition activates stat signaling to increase breast cancer immunogenicity via MHC-I expression. Cancer Drug Resist. 2020;3:603–12.
Dennison L, Ruggieri A, Mohan A, Leatherman J, Cruz K, Woolman S, et al. Context-dependent immunomodulatory effects of MEK inhibition are enhanced with T-cell agonist therapy. Cancer Immunol Res. 2021;9:1187–201.
Stopfer LE, Rettko NJ, Leddy O, Mesfin JM, Brown E, Winski S, et al. MEK inhibition enhances presentation of targetable MHC-I tumor antigens in mutant melanomas. Proc Natl Acad Sci USA. 2022;119:e2208900119.
Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602–9.
Chen YC, Chuang CH, Miao ZF, Yip KL, Liu CJ, Li LH, et al. Gut microbiota composition in chemotherapy and targeted therapy of patients with metastatic colorectal cancer. Front Oncol. 2022;12:955313.
He Y, Fu L, Li Y, Wang W, Gong M, Zhang J, et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab. 2021;33:988–1000.e7.
Trivieri N, Pracella R, Cariglia MG, Panebianco C, Parrella P, Visioli A, et al. BRAF(V600E) mutation impinges on gut microbial markers defining novel biomarkers for serrated colorectal cancer effective therapies. J Exp Clin Cancer Res. 2020;39:285.
Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–7.
Chen X, Lu Q, Zhou H, Liu J, Nadorp B, Lasry A, et al. A membrane-associated MHC-I inhibitory axis for cancer immune evasion. Cell. 2023;186:3903–20.e21.
Wang Y, Wang X, Cui X, Zhuo Y, Li H, Ha C, et al. Oncoprotein SND1 hijacks nascent MHC-I heavy chain to er-associated degradation, leading to impaired CD8(+) T cell response in tumor. Sci Adv. 2020;6:eaba5412.
Erkes DA, Cai W, Sanchez IM, Purwin TJ, Rogers C, Field CO, et al. Mutant braf and mek inhibitors regulate the tumor immune microenvironment via pyroptosis. Cancer Discov. 2020;10:254–69.
Zhang P, Kawakami H, Liu W, Zeng X, Strebhardt K, Tao K, et al. Targeting CDK1 and MEK/ERK overcomes apoptotic resistance in braf-mutant human colorectal cancer. Mol Cancer Res. 2018;16:378–89.
Gu SS, Zhang W, Wang X, Jiang P, Traugh N, Li Z, et al. Therapeutically increasing MHC-I expression potentiates immune checkpoint blockade. Cancer Discov. 2021;11:1524–41.
Meissner TB, Li A, Biswas A, Lee KH, Liu YJ, Bayir E, et al. NLR family member NLRC5 is a transcriptional regulator of MHC class I genes. Proc Natl Acad Sci USA. 2010;107:13794–9.
Yoshihama S, Vijayan S, Sidiq T, Kobayashi KS. NLRC5/CITA: a key player in cancer immune surveillance. Trends Cancer. 2017;3:28–38.
Yang Y, Bedford MT. Protein arginine methyltransferases and cancer. Nat Rev Cancer. 2013;13:37–50.
Sapir T, Shifteh D, Pahmer M, Goel S, Maitra R. Protein arginine methyltransferase 5 (PRMT5) and the ERK1/2 & PI3K pathways: a case for PRMT5 inhibition and combination therapies in cancer. Mol Cancer Res. 2021;19:388–94.
Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. 2019;16:690–704.
Wong-Rolle A, Wei HK, Zhao C, Jin C. Unexpected guests in the tumor microenvironment: microbiome in cancer. Protein Cell. 2021;12:426–35.
Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–52.
Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 2016;167:829–42.e13.
Hernández VM, Arteaga A, Dunn MF. Diversity, properties and functions of bacterial arginases. FEMS Microbiol Rev. 2021;45:fuab034.
Charoentong P, Finotello F, Angelova M, Mayer C, Efremova M, Rieder D, et al. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 2017;18:248–62.
Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, Gupta S, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581:100–05.
Jalan M, Sharma A, Pei X, Weinhold N, Buechelmaier ES, Zhu Y, et al. RAD52 resolves transcription-replication conflicts to mitigate R-loop induced genome instability. Nat Commun. 2024;15:7776.
Hung S, Saiakhova A, Faber ZJ, Bartels CF, Neu D, Bayles I, et al. Mismatch repair-signature mutations activate gene enhancers across human colorectal cancer epigenomes. Elife. 2019;8:e40760.
Liu L, Ba Y, Yang S, Zuo A, Liu S, Zhang Y, et al. FOS-driven inflammatory CAFs promote colorectal cancer liver metastasis via the SFRP1-FGFR2-HIF1 axis. Theranostics. 2025;15:4593–613.
Li D, Bentley C, Anderson A, Wiblin S, Cleary KLS, Koustoulidou S, et al. Development of a T-cell receptor mimic antibody against wild-type p53 for cancer immunotherapy. Cancer Res. 2017;77:2699–711.
Malik A, Sharma D, Aguirre-Gamboa R, McGrath S, Zabala S, Weber C, et al. Epithelial IFNγ signalling and compartmentalized antigen presentation orchestrate gut immunity. Nature. 2023;623:1044–52.
Yang Q, Wang B, Zheng Q, Li H, Meng X, Zhou F, et al. A review of gut microbiota-derived metabolites in tumor progression and cancer therapy. Adv Sci. 2023;10:e2207366.
Tharp KM, Kersten K, Maller O, Timblin GA, Stashko C, Canale FP, et al. Tumor-associated macrophages restrict CD8+ T cell function through collagen deposition and metabolic reprogramming of the breast cancer microenvironment. Nat Cancer. 2024;5:1045–62.
Wan X, Eguchi A, Sakamoto A, Fujita Y, Yang Y, Qu Y, et al. Impact of broad-spectrum antibiotics on the gut-microbiota-spleen-brain axis. Brain Behav Immun Health. 2023;27:100573.
Smith AB, Jenior ML, Keenan O, Hart JL, Specker J, Abbas A, et al. Enterococci enhance clostridioides difficile pathogenesis. Nature. 2022;611:780–86.
Acknowledgements
We thank Professor Wende Li from Guangdong Laboratory Animals Monitoring Institute for kindly providing OT-I mice.
Funding
This work was supported by National Natural Science Foundation of China (82172337); Guangdong Basic and Applied Basic Research Foundation (2021A1515012530, 2022A1515140001, 2023A1515012377); Guangdong Provincial Clinical Research Center for Digestive Diseases (2020B1111170004); The Announcement and Recruitment Project of the Sixth Affiliated Hospital of Sun Yat-sen University (2022JBGS08); National Key Clinical Discipline.
Author information
Authors and Affiliations
Contributions
JZ, HD and LL conducted the experiments, prepared the figures, drafted the manuscript. LH, JC and WL provided technical support and suggestions for the manuscript. JL, YS, MS and YF participated in the analysis and interpretation of data. EN and CL revised the manuscript. HL, XY and CW designed the work, supervised the project and revised the manuscript. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Written informed consent was obtained from the patients, and the protocol for using human blood samples was approved by the Institutional Review Board of the Sixth Affiliated Hospital of Sun Yat-sen University (2018ZSLYEC-008). The animal experiments in this study were evaluated and approved by the Institutional Animal Care and Use Committee (IACUC, NO: IACUC- 2022032701, 2021122201) of The Sixth Affiliated Hospital, Sun Yat‐sen University. All analyses were conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Dong, H., Liang, L. et al. Targeting gut microbiota and arginase boosts MEK inhibitors’ enhancement of antitumour immunity via MHC-I upregulation in colorectal cancer. Br J Cancer 133, 809–822 (2025). https://doi.org/10.1038/s41416-025-03106-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-03106-1