Abstract
Background
Docetaxel is the most common chemotherapy regimen for several neoplasms, including advanced OSCC (Oral Squamous Cell Carcinoma). Unfortunately, chemoresistance leads to relapse and adverse disease outcomes.
Methods
We performed CRISPR-based kinome screening to identify potential players of Docetaxel resistance. Immunohistochemistry was performed to examine the expression profile of the target gene across tumour tissues. Global transcriptome analysis was performed to determine the molecular mechanism underlying Docetaxel resistance. NEK9 kinase assay was performed to identify a putative kinase inhibitor.
Results
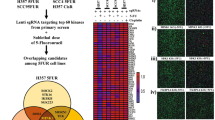
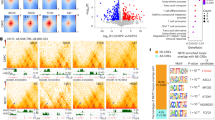
Upon conducting CRISPR-based kinome screening, Never In Mitosis Gene-A Related Kinase-9 (NEK9) was identified as a major player of Docetaxel resistance in OSCC, prostate, and pancreatic cancer lines. NEK9 expression was found to be upregulated in chemotherapy non-responder OSCC patients as compared to responders. NEK9 ablation restores Docetaxel-induced cell death in chemoresistant cells. Mechanistically, we found that NEK9 deletion upregulates Transducin-like enhancer protein 3 (TLE3), which in turn represses Wnt signalling. Fostamatinib was identified as a potent NEK9 inhibitor that overcomes Docetaxel resistance.
Conclusions
Our study demonstrated that NEK9 plays an important role in Docetaxel resistance. The novel combination of NEK9 inhibitor Fostamatinib and Docetaxel needs further clinical investigation in advanced OSCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
RNA sequencing of DU145 (a human prostate cancer cell line) NEK9 genetically knocked out cell line compared to its wildtype parental counterpart has been updated in Array Express, and the assigned ArrayExpress accession is “E-MTAB-14795”. All data are available in the main text or the supplementary materials. The URL to the deposited RNA sequencing dataset is provided below: https://www.ebi.ac.uk/fg/annotare/#list:completed.
References
Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–7.
Dumontet C, Jordan MA. Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov. 2010;9:790–803.
Tan Q, Liu X, Fu X, Li Q, Dou J, Zhai G. Current development in nanoformulations of docetaxel. Expert Opin Drug Deliv. 2012;9:975–90.
Gau M, Karabajakian A, Reverdy T, Neidhardt EM, Fayette J. Induction chemotherapy in head and neck cancers: Results and controversies. Oral Oncol. 2019;95:164–9.
Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–7.
Mhaidat NM, Zhang XD, Jiang CC, Hersey P. Docetaxel-induced apoptosis of human melanoma is mediated by activation of c-Jun NH2-terminal kinase and inhibited by the mitogen-activated protein kinase extracellular signal-regulated kinase 1/2 pathway. Clin Cancer Res. 2007;13:1308–14.
Hsieh CY, Lin CC, Chang WC. Taxanes in the treatment of head and neck squamous cell carcinoma. Biomedicines. 2023;11:2887.
Jin L, Chun J, Pan C, Li D, Lin R, Alesi GN, et al. MAST1 drives cisplatin resistance in human cancers by rewiring cRaf-independent MEK activation. Cancer cell. 2018;34:315–30.e7.
Mohanty S, Mohapatra P, Shriwas O, Ansari SA, Priyadarshini M, Priyadarsini S, et al. CRISPR-based kinome-screening revealed MINK1 as a druggable player to rewire 5FU-resistance in OSCC through AKT/MDM2/p53 axis. Oncogene. 2022;41:4929–40.
Panchal NK, Evan Prince S. The NEK family of serine/threonine kinases as a biomarker for cancer. Clin Exp Med. 2023;23:17–30.
Fry AM, O’Regan L, Sabir SR, Bayliss R. Cell cycle regulation by the NEK family of protein kinases. J Cell Sci. 2012;125:4423–33.
Belham C, Roig J, Caldwell JA, Aoyama Y, Kemp BE, Comb M, et al. A mitotic cascade of NIMA family kinases. Nercc1/Nek9 activates the Nek6 and Nek7 kinases. J Biol Chem. 2003;278:34897–909.
Lu G, Du R, Dong J, Sun Y, Zhou F, Feng F, et al. Cancer associated fibroblast derived SLIT2 drives gastric cancer cell metastasis by activating NEK9. Cell Death Dis. 2023;14:421.
Lu G, Tian S, Sun Y, Dong J, Wang N, Zeng J, et al. NEK9, a novel effector of IL-6/STAT3, regulates metastasis of gastric cancer by targeting ARHGEF2 phosphorylation. Theranostics. 2021;11:2460–74.
Maji S, Shriwas O, Samal SK, Priyadarshini M, Rath R, Panda S, et al. STAT3- and GSK3beta-mediated Mcl-1 regulation modulates TPF resistance in oral squamous cell carcinoma. Carcinogenesis. 2019;40:173–83.
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–5.
Kalita B, Sahu S, Bharadwaj A, Panneerselvam L, Martinez-Cebrian G, Agarwal M, et al. The Wnt-pathway corepressor TLE3 interacts with the histone methyltransferase KMT1A to inhibit differentiation in Rhabdomyosarcoma. Oncogene. 2024;43:524–38.
Wishart DS, Knox C, Guo AC, Shrivastava S, Hassanali M, Stothard P, et al. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006;34:D668–72.
Phadke M, Remsing Rix LL, Smalley I, Bryant AT, Luo Y, Lawrence HR, et al. Dabrafenib inhibits the growth of BRAF-WT cancers through CDK16 and NEK9 inhibition. Mol Oncol. 2018;12:74–88.
Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N. Engl J Med. 2007;357:1695–704.
Ju H, Wei D, Wu Y, Liu Y, Ding Q, Rui M, et al. A pilot study of camrelizumab with docetaxel and cisplatin for the first line treatment in recurrent/metastatic oral squamous cell carcinoma. MedComm. 2023;4:e312.
Yvon AM, Wadsworth P, Jordan MA. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol Biol Cell. 1999;10:947–59.
Hernandez-Vargas H, Palacios J, Moreno-Bueno G. Molecular profiling of docetaxel cytotoxicity in breast cancer cells: uncoupling of aberrant mitosis and apoptosis. Oncogene. 2007;26:2902–13.
Hou J, Hsu JM, Hung MC. Molecular mechanisms and functions of pyroptosis in inflammation and antitumor immunity. Mol Cell. 2021;81:4579–90.
Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–21.
Demarco B, Grayczyk JP, Bjanes E, Le Roy D, Tonnus W, Assenmacher CA, et al. Caspase-8-dependent gasdermin D cleavage promotes antimicrobial defense but confers susceptibility to TNF-induced lethality. Sci Adv. 2020;6:eabc3465.
Wang Y, Gao W, Shi X, Ding J, Liu W, He H, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103.
Erkes DA, Cai W, Sanchez IM, Purwin TJ, Rogers C, Field CO, et al. Mutant BRAF and MEK inhibitors regulate the tumor immune microenvironment via pyroptosis. Cancer Discov. 2020;10:254–69.
Su L, Chen Y, Huang C, Wu S, Wang X, Zhao X, et al. Targeting Src reactivates pyroptosis to reverse chemoresistance in lung and pancreatic cancer models. Sci Transl Med. 2023;15:eabl7895.
Liu L, Zhang Y, Wong CC, Zhang J, Dong Y, Li X, et al. RNF6 promotes colorectal cancer by activating the Wnt/β-catenin pathway via ubiquitination of TLE3. Cancer Res. 2018;78:1958–71.
Jiménez-Guerrero R, Belmonte-Fernández A, Flores ML, González-Moreno M, Pérez-Valderrama B, Romero F, et al. Wnt/β-catenin signaling contributes to paclitaxel resistance in bladder cancer cells with cancer stem cell-like properties. Int J Mol Sci. 2021;23:450.
Kornspan D, Smith Y, Nechushtan H. Differential functions of TLE1 and TLE3 depending on a specific phosphorylation site. Biochem Biophys Res Commun. 2021;545:164–70.
Braselmann S, Taylor V, Zhao H, Wang S, Sylvain C, Baluom M, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J Pharm Exp Ther. 2006;319:998–1008.
Clemens GR, Schroeder RE, Magness SH, Weaver EV, Lech JW, Taylor VC, et al. Developmental toxicity associated with receptor tyrosine kinase Ret inhibition in reproductive toxicity testing. Birth Defects Res A Clin Mol Teratol. 2009;85:130–6.
Bussel J, Arnold DM, Grossbard E, Mayer J, Trelinski J, Homenda W, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: Results of two phase 3, randomized, placebo-controlled trials. Am J Hematol. 2018;93:921–30.
Polak A, Bialopiotrowicz E, Krzymieniewska B, Wozniak J, Stojak M, Cybulska M, et al. SYK inhibition targets acute myeloid leukemia stem cells by blocking their oxidative metabolism. Cell Death Dis. 2020;11:956.
Rohila D, Park IH, Pham TV, Weitz J, Hurtado de Mendoza T, Madheswaran S, et al. Syk inhibition reprograms tumor-associated macrophages and overcomes gemcitabine-induced immunosuppression in pancreatic ductal adenocarcinoma. Cancer Res. 2023;83:2675–89.
Park SR, Speranza G, Piekarz R, Wright JJ, Kinders RJ, Wang L, et al. A multi-histology trial of fostamatinib in patients with advanced colorectal, non-small cell lung, head and neck, thyroid, and renal cell carcinomas, and pheochromocytomas. Cancer Chemother Pharm. 2013;71:981–90.
Acknowledgements
SAA is a UGC-SRF, SM is UGC-SRF, and PM is CSIR-SRF. We acknowledge the Institute of Life Sciences, Bhubaneswar, intramural support and DBT BT/ INF/22/SP28293/2018 (for imaging facility).
Funding
This work is funded by Anusandhan National Research Foundation (ANRF: CRG/2022/003427) and Indian Council of Medical Research (ICMR: 2021-10182/GENOMICS/ADHOC-BMS).
Author information
Authors and Affiliations
Contributions
Conceptualization: RD, SAA. Methodology: SAA, SM, PM, MS, RKS, SR. Investigation: RD, RR, DM, SKDM. Visualization: RD, SAA, SD. Supervision: RD. Writing-original draft: RD, SAA.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All the authors have given their consent for the publication.
Ethics approval and consent to participate
The study was approved by the Institute Review Board and Human Ethics Committees (HEC) of the Institute of Life Sciences, Bhubaneswar (120/HEC/23) and All India Institute of Medical Sciences, Bhubaneswar (T/EMF/ Surg.Onco/19/03). All study participants provided written informed consent and agreed to participate in this study. Animal-related experiments were conducted as per the ARRIVE reporting guidelines 2.0 and in accordance with the protocol approved by the Institutional Animal Ethics Committee of Institute of Life Sciences, Bhubaneswar (ILS/IAEC-289-AH/ NOV-22). The Institutional Biosafety Committee (IBSC) approved all related experiments.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ansari, S.A., Mohanty, S., Mohapatra, P. et al. NEK9-mediated Wnt signalling repressor TLE3 rewires Docetaxel resistance in cancer cells by inducing pyroptosis. Br J Cancer 133, 1428–1440 (2025). https://doi.org/10.1038/s41416-025-03148-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-03148-5