Abstract
Background
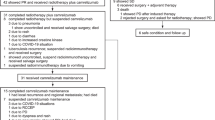
Locally advanced nasopharyngeal carcinoma (LA-NPC) has a heterogeneous prognosis, with approximately one-fourth of patients experiencing poor outcomes. Studies have explored the application of induction chemoimmunotherapy followed by chemoradiotherapy, but its efficacy was controversial.
Methods
The protocol was registered in the Prospective Register of Systematic Reviews (PROSPERO, CRD42024619387). The primary outcome measures were objective response rate (ORR), complete response rate (CRR), and the incidence of treatment-related adverse events (TRAEs). Meta-analysis was performed using Cochrane Collaboration Review Manager 5.4.1 and Meta-Analyst Beta 3.13 statistical software.
Results
The meta-analysis involving 1680 patients with LA-NPC from 7 studies showed that the induction chemoimmunotherapy group had significantly better ORR (odds ratio[OR] = 2.03, 95% confidence interval [CI]:1.44–2.86, P < 0.01), and CRR (OR = 2.61, 95% CI:1.55–4.38, P < 0.01) than the induction chemotherapy group. The pooled ORR and CRR of induction chemoimmunotherapy were 92.7% (95% CI: 90.7–94.7%) and 24.3% (95% CI: 15.2–33.6%), respectively. There was no significant difference of TRAEs between induction chemotherapy group and induction chemoimmunotherapy group (OR = 1.13; 95% CI: 0.92–1.39, P = 0.23).
Conclusions
Induction chemoimmunotherapy could be a promising induction treatment option for LA-NPC patients, improving ORR and CRR with an acceptable safety profile. However, due to limitations in this meta-analysis, further large-scale, well-designed clinical trials are required to validate these results and optimise treatment strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80.
Su ZY, Siak PY, Lwin YY, Cheah SC. Epidemiology of nasopharyngeal carcinoma: current insights and future outlook. Cancer Metastasis Rev. 2024;43:919–39.
Juarez-Vignon Whaley JJ, Afkhami M, Sampath S, Amini A, Bell D, Villaflor VM. Early stage and locally advanced nasopharyngeal carcinoma treatment from present to future: where are we and where are we going? Curr Treat Options Oncol. 2023;24:845–66.
Liu GY, Ye YF, Jiang YF, Chen GJ, Xia WX, Huang YS, et al. Nab-paclitaxel, cisplatin, and capecitabine versus cisplatin and gemcitabine as first line chemotherapy in patients with recurrent or metastatic nasopharyngeal carcinoma: randomised phase 3 clinical trial. BMJ. 2024;385:e077890.
Yang Y, Qu S, Li J, Hu C, Xu M, Li W, et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2021;22:1162–74.
Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen J, et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med. 2021;27:1536–43.
Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen J, et al. Toripalimab plus chemotherapy for recurrent or metastatic nasopharyngeal carcinoma: the JUPITER-02 randomized clinical trial. JAMA. 2023;330:1961–70.
Yang Y, Pan J, Wang H, Zhao Y, Qu S, Chen N, et al. Tislelizumab plus chemotherapy as first-line treatment for recurrent or metastatic nasopharyngeal cancer: a multicenter phase 3 trial (RATIONALE-309). Cancer Cell. 2023;41:1061–72.e4.
Cai M, Wang Y, Ma H, Yang L, Xu Z. Advances and challenges in immunotherapy for locally advanced nasopharyngeal carcinoma. Cancer Treat Rev. 2024;131:102840.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5.
Zheng B, Huang Z, Liu W, Zhao D, Xu X, Xiao S, et al. A real-world evaluation of tislelizumab in patients with head and neck cancer. Transl Cancer Res. 2024;13:808–18.
Kang M, Ma D, Gao J, Wang F, Yu B, Lu Y, et al. Endostar combined with concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone for locoregionally advanced nasopharyngeal carcinoma (LA-NPC): a phase III, prospective, randomised-controlled, multicenter clinical trial. J Clin Oncol. 2024;42:6002.
Liu SL, Li XY, Yang JH, Wen DX, Guo SS, Liu LT, et al. Neoadjuvant and adjuvant toripalimab for locoregionally advanced nasopharyngeal carcinoma: a randomised, single-centre, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2024;25:1563–75.
Ma J, Sun Y, Liang Y-L, Liu X, Shen L, Hu W, et al. Adjuvant PD-1 blockade with camrelizumab in high-risk locoregionally advanced nasopharyngeal carcinoma (DIPPER): a multicenter, open-label, phase 3, randomized controlled trial. LBA. 2024;42:LBA6000.
Yuan Y, Tian Y, Zhong H, Zheng R, Jie L, Zhang J, et al. Neoadjuvant docetaxel-cisplatin followed by concurrent chemoradiotherapy and adjuvant tislelizumab for locally advanced nasopharyngeal carcinoma: a multicenter, single-arm, phase II trial. J Clin Oncol. 2024;42:e18047.
Wang X, Han F, Huang Y, Xu C, Mao Y, Lin L, et al. 920P Envafolimab plus chemoradiotherapy for locally advanced <strong>nasopharyngeal carcinoma</strong> (NPC), a prospective, single-armed phase II trial. Ann Oncol. 2023;34:S581.
Fu H, Chen Z, Chen J, Zhang S. Efficacy and safety of neoadjuvant immunotherapy combined with sandwich chemoradiotherapy in locally advanced nasopharyngeal carcinoma: a retrospective study. Oncol Targets Ther. 2024;17:1145–55.
Liang H, Jiang YF, Liu GY, Wang L, Wang JW, Lu N, et al. Camrelizumab and apatinib plus induction chemotherapy and concurrent chemoradiotherapy in stage N3 nasopharyngeal carcinoma: a phase 2 clinical trial. Nat Commun. 2024;15:1029.
Chen Q-Y, Mai H-Q, Tang L-Q, Luo M, Zhao C, Mo H-Y, et al. Neoadjuvant chemotherapy plus tislelizumab followed by concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: a single-arm, phase II trial. J Clin Oncol. 2022;40:6068.
Liu X, Zhang Y, Yang KY, Zhang N, Jin F, Zou GR, et al. Induction-concurrent chemoradiotherapy with or without sintilimab in patients with locoregionally advanced nasopharyngeal carcinoma in China (CONTINUUM): a multicentre, open-label, parallel-group, randomised, controlled, phase 3 trial. Lancet. 2024;403:2720–31.
Mai H-Q, Liu SL, Chen Q-Y, Tang L-Q, Jin F, Guo L, et al. Tislelizumab versus placebo combined with induction chemotherapy followed by concurrent chemoradiotherapy and adjuvant tislelizumab or placebo for locoregionally advanced nasopharyngeal carcinoma: Interim analysis of a multicenter, randomized, placebo-controlled, double-blind, phase 3 trial. J Clin Oncol. 2024;42:6001.
Jin YN, Qiang MY, Wang Y, Lin YJ, Jiang RW, Cao WW, et al. The efficacy and safety of adding PD-1 blockade to induction chemotherapy and concurrent chemoradiotherapy (IC-CCRT) for locoregionally advanced nasopharyngeal carcinoma: an observational, propensity score-matched analysis. Cancer Immunol Immunother. 2024;73:125.
Yao Y, Ouyang Q, Wang S, Li K, Luo Q, Qiu L, et al. Incorporation of PD-1 blockade into induction chemotherapy improved tumor response in patients with locoregionally advanced nasopharyngeal carcinoma in a retrospective patient cohort. Oral Oncol. 2024;154:106867.
Yu YF, Lu GZ, Wang RJ, Song YK, Wu SG. Additional PD-1 inhibitor improves complete response to induction chemotherapy in locally advanced nasopharyngeal carcinoma. Front Immunol. 2024;15:1415246.
He J, Luo G, Liu S, Chen L, Chen Z, Zhang B, et al. Tislelizumab plus neoadjuvant chemotherapy and concurrent chemoradiotherapy versus neoadjuvant chemotherapy and concurrent chemoradiotherapy for locally advanced nasopharyngeal carcinoma: a retrospective study. Transl Oncol. 2024;48:102058.
Wu K, Li Q, Luo X, Wei X, Wang L, Li YS. 455P A real-world study: Induction chemoimmunotherapy versus induction chemotherapy followed by concurrent chemoradiotherapy in nasopharyngeal carcinoma patients with stage IVa. Ann Oncol. 2024;35:S1572.
Bossi P, Gurizzan C, Chan A. Immunotherapy for nasopharyngeal carcinoma: the earlier the better. JAMA. 2023;330:1954–5.
Huang H, Yao Y, Deng X, Huang Z, Chen Y, Wang Z, et al. Immunotherapy for nasopharyngeal carcinoma: Current status and prospects (Review). Int J Oncol. 2023;63:97.
Yan K, Lim DW, Ma B. Progress in the clinical development of investigational systemic agents for recurrent and metastatic nasopharyngeal carcinoma. Expert Opin Investig Drugs. 2024;33:1019–28.
Liu X, Shen H, Zhang L, Huang W, Zhang S, Zhang B. Immunotherapy for recurrent or metastatic nasopharyngeal carcinoma. NPJ Precis Oncol. 2024;8:101.
Juarez-Vignon Whaley JJ, Afkhami M, Onyshchenko M, Massarelli E, Sampath S, Amini A, et al. Recurrent/metastatic nasopharyngeal carcinoma treatment from present to future: where are we and where are we heading? Curr Treat Options Oncol. 2023;24:1138–66.
Yu Z, Hong S, Yu H, Zhang X, Li Z, Chen P, et al. Efficacy and safety of immune checkpoint inhibitors in the treatment of recurrent or metastatic nasopharyngeal carcinoma: a systematic review and meta-analysis. Chin Med J. 2024;138:531–39.
Wang BC, Kuang BH, Liu XX, Lin GH, Liu Q. Induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: a systematic review and meta-analysis. Front Oncol. 2022;12:927510.
Sordo-Bahamonde C, Lorenzo-Herrero S, Gonzalez-Rodriguez AP, Martinez-Perez A, Rodrigo JP, Garcia-Pedrero JM, et al. Chemo-immunotherapy: a new trend in cancer treatment. Cancers. 2023;15:2912.
Principe DR, Kamath SD, Korc M, Munshi HG. The immune modifying effects of chemotherapy and advances in chemo-immunotherapy. Pharm Ther. 2022;236:108111.
Acknowledgements
This work was partially presented at the American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL (May 30-June 3, 2025).
Funding
This study was supported by grants from the Medical Scientific Research Foundation of Guangdong Province (Grant No.B2023476), the High-level Hospital Construction Project of Heyuan People’s Hospital (Grant No.YNKT202203, Grant No.YNKT202218), Guangdong Basic and Applied Basic Research Foundation (Grant No. 2024A1515030265), and Youth science and technology innovation talent of Guangdong Special Branch Plan (Grant No.KY012024253).
Author information
Authors and Affiliations
Contributions
KPW, LC, and YSL conceived the study and contributed to study design and data interpretation. KPW, XQL, HCY, MCJ, XZ, and YZL did the literature search, data extraction, and data analysis. XQL and QQL accessed and verified the data. KPW, XQL, and QQL wrote the manuscript. YL, HJY, and DT provided material support. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This meta-analysis was performed by following the PRISMA guideline. Ethical approval was not requested because this study retrieved and synthesised data from already published studies.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, KP., Luo, XQ., Li, QQ. et al. Efficacy and safety of induction immunotherapy plus chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: a meta-analysis. Br J Cancer 133, 1518–1525 (2025). https://doi.org/10.1038/s41416-025-03169-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-03169-0