Abstract
Objective
Concurrent chemoradiotherapy (CCRT) is an important treatment for patients with locally advanced esophageal squamous cell carcinoma (ESCC). There is still a lack of reliable means to predict efficacy, prognosis and hematologic toxicity.
Design
We analyzed 127 serum samples before CCRT and 93 serum samples after CCRT from 127 ESCC patients via metabolomics by GC-MS. Combined with Olink proteomics, we constructed models to predict response and survival through machine learning. Multiple linear regression was used to construct hematologic toxicity prediction models. In combination with the proteomics of ESCC, metabolic changes were studied.
Results
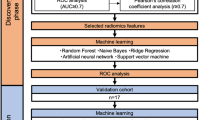
A prediction model for the efficacy to CCRT was established via serum metabolomics and proteomics (Train, CR/nCR = 28/50, AUC = 0.9848, 95% CI = 0.9639–1.0000; Test, CR/nCR = 17/15, AUC = 0.8854, 95% CI = 0.7800–0.9908). A survival prediction model was established (n = 109, C-index = 0.7640, 95% CI = 0.7140–0.8140). Linear models for predicting hematologic toxicity were constructed (n = 111, R > 0.7). L-serine is important for the prognosis of patients with ESCC treated with CCRT, and SHMT2 is a key protein in serine metabolism that affects the efficacy of CCRT.
Conclusion
The combination of serum metabolomics with proteomics can effectively predict the prognosis and hematologic toxicity, which can provide important data for patients to choose treatment methods.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data for this study were extracted from published literature. Pertinent data are presented in both the manuscript and its Supplementary Material file.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2016. Journal Natl Cancer Cent. 2022;2:1–9.
Chen C, Chen J, Luo T, Wang S, Guo H, Zeng C, et al. Late Toxicities, Failure Patterns, Local Tumor Control, and Survival of Esophageal Squamous Cell Carcinoma Patients After Chemoradiotherapy With a Simultaneous Integrated Boost: A 5-Year Phase II Study. Front Oncol. 2021;11:738936.
Yao Y, Lu J, Qin Z, Li N, Ma J, Yao N, et al. High-dose versus standard-dose radiotherapy in concurrent chemoradiotherapy for inoperable esophageal cancer: A systematic review and meta-analysis. Radiother Oncol. 2023;184:109700.
Zhu H, Lu X, Jiang J, Lu J, Sun X, Zuo Y. Radiotherapy Combined With Concurrent Nedaplatin-Based Chemotherapy for Stage II-III Esophageal Squamous Cell Carcinoma. Dose-response : a Publ Int Hormesis Soc. 2022;20:15593258221076720.
Hurmuzlu M, Monge OR, Smaaland R, Viste A. High-dose definitive concomitant chemoradiotherapy in non-metastatic locally advanced esophageal cancer: toxicity and outcome. Dis Esophagus. 2010;23:244–52.
Sato Y, Motoyama S, Saito H, Minamiya Y. Novel Candidate Biomarkers of Chemoradiosensitivity in Esophageal Squamous Cell Carcinoma: A Systematic Review. Eur Surg Res. 2016;56:141–53.
Song T, Liang X, Fang M, Wu S. High-dose versus conventional-dose irradiation in cisplatin-based definitive concurrent chemoradiotherapy for esophageal cancer: a systematic review and pooled analysis. Expert Rev Anticancer Ther. 2015;15:1157–69.
Chen YH, Lu HI, Lo CM, Wang YM, Chou SY, Hsiao CC, et al. The clinical outcomes of locally advanced cervical esophageal squamous cell carcinoma patients receiving curative concurrent chemoradiotherapy: a population-based propensity score-matched analysis. Cancers (Basel). 2019;11:451.
Kang J, Chang JY, Sun X, Men Y, Zeng H, Hui Z. Role of Postoperative Concurrent Chemoradiotherapy for Esophageal Carcinoma: A meta-analysis of 2165 Patients. J Cancer. 2018;9:584–93.
Bohlius J, Bohlke K, Castelli R, Djulbegovic B, Lustberg MB, Martino M, et al. Management of Cancer-Associated Anemia With Erythropoiesis-Stimulating Agents: ASCO/ASH Clinical Practice Guideline Update. J Clin Oncol. 2019;37:1336–51.
Crawford J, Becker PS, Armitage JO, Blayney DW, Chavez J, Curtin P, et al. Myeloid Growth Factors, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:1520–41.
Lyu J, Li T, Wang Q, Li F, Diao P, Liu L, et al. Outcomes of concurrent chemoradiotherapy versus chemotherapy alone for stage IV esophageal squamous cell carcinoma: a retrospective controlled study. Radiat Oncol. 2018;13:233.
Wang PP, Song X, Zhao XK, Wei MX, Gao SG, Zhou FY, et al. Serum Metabolomic Profiling Reveals Biomarkers for Early Detection and Prognosis of Esophageal Squamous Cell Carcinoma. Front Oncol. 2022;12:790933.
Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308.
Yu M, Wen W, Yi X, Zhu W, Aa J, Wang G. Plasma Metabolomics Reveals Diagnostic Biomarkers and Risk Factors for Esophageal Squamous Cell Carcinoma. Front Oncol. 2022;12:829350.
Cui Y, Yang D, Wang W, Zhang L, Liu H, Ma S, et al. Nicotinamide N-methyltransferase decreases 5-fluorouracil sensitivity in human esophageal squamous cell carcinoma through metabolic reprogramming and promoting the Warburg effect. Mol Carcinog. 2020;59:940–54.
Qie S, Yoshida A, Parnham S, Oleinik N, Beeson GC, Beeson CC, et al. Targeting glutamine-addiction and overcoming CDK4/6 inhibitor resistance in human esophageal squamous cell carcinoma. Nat Commun. 2019;10:1296.
Cruz-Bermúdez A, Laza-Briviesca R, Vicente-Blanco RJ, García-Grande A, Coronado MJ, Laine-Menéndez S, et al. Cisplatin resistance involves a metabolic reprogramming through ROS and PGC-1α in NSCLC which can be overcome by OXPHOS inhibition. Free Radic Biol Med. 2019;135:167–81.
Xu J, Chen Y, Zhang R, Song Y, Cao J, Bi N, et al. Global and targeted metabolomics of esophageal squamous cell carcinoma discovers potential diagnostic and therapeutic biomarkers. Mol Cell Proteom. 2013;12:1306–18.
Fujigaki S, Nishiumi S, Kobayashi T, Suzuki M, Iemoto T, Kojima T, et al. Identification of serum biomarkers of chemoradiosensitivity in esophageal cancer via the targeted metabolomics approach. Biomark Med. 2018;12:827–40.
Kerzeli IK, Kostakis A, Türker P, Malmström PU, Hemdan T, Mezheyeuski A, et al. Elevated levels of MMP12 sourced from macrophages are associated with poor prognosis in urothelial bladder cancer. BMC Cancer. 2023;23:605.
Davies MPA, Sato T, Ashoor H, Hou L, Liloglou T, Yang R, et al. Plasma protein biomarkers for early prediction of lung cancer. EBioMedicine. 2023;93:104686.
Li ZM, Liu W, Chen XL, Wu WZ, Xu XE, Chu MY, et al. Construction and validation of classification models for predicting the response to concurrent chemo-radiotherapy of patients with esophageal squamous cell carcinoma based on multi-omics data. Clinics Res Hepatol Gastroenterol. 2024;48:102318.
Wang D, Wang X, Kong J, Wu J, Lai M. GC-MS-Based metabolomics discovers a shared serum metabolic characteristic among three types of epileptic seizures. Epilepsy Res. 2016;126:83–9.
Wang D, Kong J, Wu J, Wang X, Lai M. GC-MS-based metabolomics identifies an amino acid signature of acute ischemic stroke. Neurosci Lett. 2017;642:7–13.
Lai M, Zhang X, Zhou D, Zhang X, Zhu M, Liu Q, et al. Integrating serum proteomics and metabolomics to compare the common and distinct features between acute aggressive ischemic stroke (APIS) and acute non-aggressive ischemic stroke (ANPIS). J Proteom. 2022;261:104581.
Liu W, Xie L, He Y-H, Wu Z-Y, Liu L-X, Bai X-F, et al. Large-scale and high-resolution mass spectrometry-based proteomics profiling defines molecular subtypes of esophageal cancer for therapeutic targeting. Nat Commun. 2021;12:4961.
Shimada Y, Imamura M, Wagata T, Yamaguchi N, Tobe T. Characterization of 21 newly established esophageal cancer cell lines. Cancer. 1992;69:277–84.
Nishihira T, Hashimoto Y, Katayama M, Mori S, Kuroki T. Molecular and cellular features of esophageal cancer cells. J Cancer Res Clin Oncol. 1993;119:441–9.
Cheung PY, Deng W, Man C, Tse WW, Srivastava G, Law S, et al. Genetic alterations in a telomerase-immortalized human esophageal epithelial cell line: Implications for carcinogenesis. Cancer Lett. 2010;293:41–51.
Zeng FM, Xie YM, Liao LD, Li LY, Chen B, Xie JJ, et al. Biological characterization of three immortalized esophageal epithelial cell lines. Molecular Med Rep. 2016;14:4802–10.
Heng J, Li Z, Liu L, Zheng Z, Zheng Y, Xu X, et al. Acetyl-CoA Acetyltransferase 2 Confers Radioresistance by Inhibiting Ferroptosis in Esophageal Squamous Cell Carcinoma. Int J Radiat Oncol, Biol, Phys. 2023;117:966–78.
Montrose DC, Saha S, Foronda M, McNally EM, Chen J, Zhou XK, et al. Exogenous and Endogenous Sources of Serine Contribute to Colon Cancer Metabolism, Growth, and Resistance to 5-Fluorouracil. Cancer Res. 2021;81:2275–88.
Falcone M, Uribe AH, Papalazarou V, Newman AC, Athineos D, Stevenson K, et al. Sensitisation of cancer cells to radiotherapy by serine and glycine starvation. Br J Cancer. 2022;127:1773–86.
Mao Y, Zhang T. Knockdown of SHMT2 enhances the sensitivity of gastric cancer cells to radiotherapy through the Wnt/β-catenin pathway. Open Life Sci. 2022;17:1249–55.
Pranzini E, Pardella E, Muccillo L, Leo A, Nesi I, Santi A, et al. SHMT2-mediated mitochondrial serine metabolism drives 5-FU resistance by fueling nucleotide biosynthesis. Cell Rep. 2022;40: 111233.
Chen J, Na R, Xiao C, Wang X, Wang Y, Yan D, et al. The loss of SHMT2 mediates 5-fluorouracil chemoresistance in colorectal cancer by upregulating autophagy. Oncogene. 2021;40:3974–88.
Wang H, Zheng Z, Zhang Y, Bian C, Bao J, Xin Y, et al. Locally advanced head and neck squamous cell carcinoma treatment efficacy and safety: a systematic review and network meta-analysis. Front Pharm. 2023;14:1269863.
Shao Y, Han X, Yu H, Liu J, Wang X, Yang Y. Evaluation of the Neutrophil-Based Inflammatory Indexes SIRI and NHR in Patients with Extensive-Stage Small Cell Lung Cancer Receiving First-Line Immune Checkpoint Inhibitors Plus Chemotherapy. Annals Clin Lab Sci. 2025;55:354–64.
Su H, Gu X, Zhang W, Lin F, Lu X, Zeng X, et al. Identification of Salivary Biomarkers in Colorectal Cancer by Integrating Olink Proteomics and Metabolomics. J Proteome Res. 2025;24:2542–52.
Kim K, Mall C, Taylor SL, Hitchcock S, Zhang C, Wettersten HI, et al. Mealtime, temporal, and daily variability of the human urinary and plasma metabolomes in a tightly controlled environment. PLoS ONE. 2014;9:e86223.
Yin P, Lehmann R, Xu G. Effects of pre-analytical processes on blood samples used in metabolomics studies. Anal Bioanal Chem. 2015;407:4879–92.
Chetwynd AJ, Dunn WB, Rodriguez-Blanco G. Collection and Preparation of Clinical Samples for Metabolomics. Adv Exp Med Biol. 2017;965:19–44.
Dayon L, Cominetti O, Affolter M. Proteomics of human biological fluids for biomarker discoveries: technical advances and recent applications. Expert Rev Proteom. 2022;19:131–51.
Sun Z, Feng D, Jiang L, Tian J, Wang J, Zhu W. Integrated proteomic and metabolomic analysis of plasma reveals regulatory pathways and key elements in thyroid cancer. Mol Omics. 2023;19:800–9.
Kiebish MA, Cullen J, Mishra P, Ali A, Milliman E, Rodrigues LO, et al. Multi-omic serum biomarkers for prognosis of disease progression in prostate cancer. J Transl Med. 2020;18:10.
Navas-Carrillo D, Rodriguez JM, Montoro-García S, Orenes-Piñero E. High-resolution proteomics and metabolomics in thyroid cancer: Deciphering novel biomarkers. Crit Rev Clin Lab Sci. 2017;54:446–57.
Lu M, Zhan X. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. Epma j. 2018;9:77–102.
Subramanian I, Verma S, Kumar S, Jere A, Anamika K. Multi-omics Data Integration, Interpretation, and Its Application. Bioinform Biol Insights. 2020;14:1177932219899051.
Acknowledgements
We are grateful to Professor Stanley Li Lin from Shantou University Medical College for assisting in proofreading and editing the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82173034, No. 82273108), the Natural Science Foundation of China–Guangdong Joint Fund (No. U0932001 and No. U1301227), the Special Fund Project for Science and Technology Innovation Strategy of Guangdong Province in 2021 (No. 210713116901849) and the Guangdong Innovative and Entrepreneurial Research Team Program (No. 2021KCXTD005).
Author information
Authors and Affiliations
Contributions
Conception and design: WZ, HH, GZ, and LX. Supervision: HH, EL, and LX. Development of methodology: WZ, GZ, ZL, MC, SY, and DW. Software: WZ. Formal analysis: WZ, and GZ. Acquisition of data: WZ, and LL. Data curation: WZ, HH, XC, ZL, MC, and SY. Visualization: WZ. Writing - original draft: WZ, HH, GZ, XC, and LX. Writing - review & editing: WZ, HH, EL, and LX. Resources: HH, GZ, LL, XC, ZL, MC, SY, and DW. Project administration: HH, and LX. Funding acquisition: HH, EL, and LX.
Corresponding authors
Ethics declarations
Competing interests
The authors of this manuscript are not current editors or Editorial Board Member of British Journal of Cancer. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this manuscript.
Ethics approval and consent to participate
Patients with ESCC were selected from our nested cohort study registered on the Clinical Trial Management Public Platform, clinical registration number: ChiCTR180001624. Informed consent was obtained from the subject(s) and/or guardian(s). All participants were informed and voluntarily participated in this study and signed an informed consent form. The study was approved by the ethics committee of the Central Hospital of Shantou (2016--026, November 4, 2016), the Ethics Committee of Shantou University Medical College (SUMC-2021--15, March 14, 2021) and the Human Genetic Resources Committee of the Ministry of Science and Technology of China ([2021] CJ1260, [2022 BC0008]. The study was performed in accordance with the Declaration of Helsinki. Ethics related to animal experiments was not addressed in this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, WZ., Huang, HC., Zhu, GH. et al. Serum multiomics prediction of prognosis and adverse reactions to concurrent chemoradiotherapy in patients with esophageal cancer. Br J Cancer 133, 1829–1843 (2025). https://doi.org/10.1038/s41416-025-03229-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-03229-5