Abstract
Background
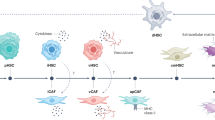
We previously reported that IL-33 released during hepatectomy contributes to cytokine-facilitated iCCA tumor growth. However, the underlying mechanisms of the tumor microenvironment remain unexplored. In this study, we aimed to elucidate the impact of IL-33 on cancer-associated fibroblasts (CAFs).
Methods
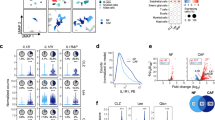
The abundance of IL-33-positive cells and alpha-SMA-positive fibroblasts (myoCAFs) was evaluated using resected specimens. Next-generation sequencing (NGS) was performed for comprehensive expression analysis. The effects of IL-33 stimulation on CAFs were investigated in vitro and in vivo using human and murine iCCA cell lines, as well as fibroblasts extracted from resected specimens.
Results
IL-33-positive cells and myoCAFs were significant risk factors for intrahepatic recurrence. NGS analysis revealed significant upregulation of various cytokines in cases with high number of IL-33-positive cells. The conditioned medium obtained from fibroblasts stimulated with IL-33 enhanced the proliferation and migration of iCCA cell lines. Among the cytokines that were increased by IL-33 stimulation in vitro, IL-6 was suspected to be the dominant. In a murine syngraft model, hepatectomy led to an increased subcutaneous tumor volume by releasing IL-33, whereas IL-6 blockade suppressed this growth.
Conclusions
This study indicated that IL-33 facilitates iCCA growth through fibroblast activation and is associated with intrahepatic recurrence.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, [S.K.], upon reasonable request. Individual participant data will not be available.
References
Yamada D, Rizvi S, Razumilava N, Bronk SF, Davila JI, Champion MD, et al. IL-33 facilitates oncogene-induced cholangiocarcinoma in mice by an interleukin-6-sensitive mechanism. Hepatology. 2015;61:1627–42.
Buettner S, van Vugt JL, IJzermans J, Groot Koerkamp B. Intrahepatic cholangiocarcinoma: current perspectives. Onco Targets Ther. 2017;10:1131–42.
Konstantinidis IT, Groot Koerkamp B, Do RK, Gonen M, Fong Y, Allen PJ, et al. Unresectable intrahepatic cholangiocarcinoma: systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer. 2016;122:758–65.
Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268–89.
Hyder O, Hatzaras I, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery. 2013;153:811–8.
Alaimo L, Moazzam Z, Brown ZJ, Endo Y, Ruzzenente A, Guglielmi A, et al. Application of hazard function to investigate recurrence of intrahepatic cholangiocarcinoma after curative-intent liver resection: a novel approach to characterize recurrence. Ann Surgical Oncol. 2023;30:1340–9.
Yang H, Wang J, Li Z, Yang Y, Yang L, Zhang Y, et al. Risk Factors and Outcomes of Early Relapse After Curative Resection of Intrahepatic Cholangiocarcinoma. Front Oncol. 2019;9:854.
Zhang Y, A Esmail, V Mazzaferro, M Abdelrahim. Newest therapies for cholangiocarcinoma: an updated overview of approved treatments with transplant oncology vision. Cancers. 14 (2022).
Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–18.
Lingel A, Weiss TM, Niebuhr M, Pan B, Appleton BA, Wiesmann C, et al. Structure of IL-33 and its interaction with the ST2 and IL-1RAcP receptors-insight into heterotrimeric IL-1 signaling complexes. Structure. 2009;17:1398–410.
Brunner SM, Schiechl G, Falk W, Schlitt HJ, Geissler EK, Fichtner-Feigl S. Interleukin-33 prolongs allograft survival during chronic cardiac rejection. Transpl Int. 2011;24:1027–39.
Peine M, Marek RM, Löhning M. IL-33 in T Cell Differentiation, Function, and Immune Homeostasis. Trends Immunol. 2016;37:321–33.
Nagaoka S, Yamada D, Eguchi H, Yokota Y, Iwagami Y, Asaoka T, et al. The blockade of interleukin-33 released by hepatectomy would be a promising treatment option for cholangiocarcinoma. Cancer Sci. 2021;112:347–58.
Liu X, Zhu L, Lu X, Bian H, Wu X, Yang W, et al. IL-33/ST2 pathway contributes to metastasis of human colorectal cancer. Biochem Biophys Res Commun. 2014;453:486–92.
Chen SF, Nieh S, Jao SW, Wu MZ, Liu CL, Chang YC, et al. The paracrine effect of cancer-associated fibroblast-induced interleukin-33 regulates the invasiveness of head and neck squamous cell carcinoma. J Pathol. 2013;231:180–9.
Yang Z, Gao X, Wang J, Xu L, Zheng Y, Xu Y. Interleukin-33 enhanced the migration and invasiveness of human lung cancer cells. OncoTargets Ther. 2018;11:843–9.
Joshi RS, Kanugula SS, Sudhir S, Pereira MP, Jain S, Aghi MK. The Role of Cancer-Associated Fibroblasts in Tumor Progression. Cancers. 2021;13.
Ramos-Vega V, Venegas Rojas B, Donoso Torres W. Immunohistochemical analysis of cancer-associated fibroblasts and podoplanin in head and neck cancer. Med Oral Patol Oral Cir Bucal. 2020;25:e268–76.
Mukai Y, Yamada D, Eguchi H, Iwagami Y, Asaoka T, Noda T, et al. Vitamin D supplementation is a promising therapy for pancreatic ductal adenocarcinoma in conjunction with current chemoradiation therapy. Ann Surg Oncol. 2018;25:1868–79.
Sirica AE. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;9:44–54.
Zou R, Jiang Q, Jin T, Chen M, Yao L, Ding H. Pan-cancer analyses and molecular subtypes based on the cancer-associated fibroblast landscape and tumor microenvironment infiltration characterization reveal clinical outcome and immunotherapy response in epithelial ovarian cancer. Front Immunol. 2022;13:956224.
Guo Z, Zhang H, Fu Y, Kuang J, Zhao B, Zhang L, et al. Cancer-associated fibroblasts induce growth and radioresistance of breast cancer cells through paracrine IL-6. Cell Death Discov. 2023;9:6.
Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214:579–96.
Lin Y-C, Huang W-Y, Lee T-Y, Chang Y-M, Chen S-F, Lin Y-S, et al. Interleukin-33-Enhanced CXCR4 Signaling Circuit Mediated by Carcinoma-Associated Fibroblasts Promotes Invasiveness of Head and Neck Cancer. Cancers. 2021;13:3442.
Feng C, Kou L, Yin P, Jing Y. Excessive activation of IL‑33/ST2 in cancer‑associated fibroblasts promotes invasion and metastasis in ovarian cancer. Oncol Lett. 2022;23.
Eguchi S, Yamada D, Kobayashi S, Sasaki K, Iwagami Y, Tomimaru Y, et al. Automated analysis for the prevalence of cancer-associated fibroblasts in resected specimens of intrahepatic cholangiocarcinoma is a simple and reliable evaluation system. Ann Surg Oncol. 2023;30:5420–8.
Kobayashi S, Werneburg NW, Bronk SF, Kaufmann SH, Gores GJ. Interleukin-6 contributes to Mcl-1 up-regulation and TRAIL resistance via an Akt-signaling pathway in cholangiocarcinoma cells. Gastroenterology. 2005;128:2054–65.
Farshidfar F, Zheng S, Gingras MC, Newton Y, Shih J, Robertson AG, et al. Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep. 2017;19:2878–80.
Shinke G, Yamada D, Eguchi H, Iwagami Y, Asaoka T, Noda T, et al. Role of histone deacetylase 1 in distant metastasis of pancreatic ductal cancer. Cancer Sci. 2018;109:2520–31.
Yamada D, Kobayashi S, Yamamoto H, Tomimaru Y, Noda T, Uemura M, et al. Role of the hypoxia-related gene, JMJD1A, in hepatocellular carcinoma: clinical impact on recurrence after hepatic resection. Ann Surg Oncol. 2012;19:S355–64.
Sha M, Jeong S, Qiu BJ, Tong Y, Xia L, Xu N, et al. Isolation of cancer-associated fibroblasts and its promotion to the progression of intrahepatic cholangiocarcinoma. Cancer Med. 2018;7:4665–77.
Chen J, Yang P, Xiao Y, Zhang Y, Liu J, Xie D, et al. Overexpression of α-sma-positive fibroblasts (CAFs) in nasopharyngeal carcinoma predicts poor prognosis. J Cancer. 2017;8:3897–902.
Cong X, Zhang Y, Zhu Z, Li S, Yin X, Zhai Z, et al. CD66b + neutrophils and α-SMA + fibroblasts predict clinical outcomes and benefits from postoperative chemotherapy in gastric adenocarcinoma. Cancer Med. 2020;9:2761–73.
Takahashi H, Sakakura K, Kudo T, Toyoda M, Kaira K, Oyama T, et al. Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget. 2017;8:8633–47.
Reivan Ortiz GG, Ciongradi CI, Chaitanya M, Narayanan J, Mohany M, Al-Rejaie SS, et al. Identification of novel candidate targets for suppressing ovarian cancer progression through IL-33/ST2 axis components using the system biology approach. Front Mol Biosci. 2023;10:1189527.
Gu D, Zhao X, Song J, Xiao J, Zhang L, Deng G, et al. Expression and clinical significance of interleukin-6 pathway in cholangiocarcinoma. Front Immunol. 2024;15.
Shinke G, Yamada D, Eguchi H, Iwagami Y, Akita H, Asaoka T, et al. The postoperative peak number of leukocytes after hepatectomy is a significant prognostic factor for cholangiocarcinoma. Mol Clin Oncol. 2019;10:531–40.
Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20:131.
Hong JC, Jones CM, Duffy JP. Comparative analysis of resection and liver transplantation for intrahepatic and Hilar cholangiocarcinoma. Arch Surg. 2011;146:683.
Choi MR, Sosman JA, Zhang B. The Janus face of IL-33 signaling in tumor development and immune escape. Cancers. 2021;13:3281.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Funding
Grant-in-Aid for Scientific Research (C), The Ministry of Education, Culture, Sports, Science and Technology (MEXT).
Author information
Authors and Affiliations
Contributions
S.E.: conception and design, acquisition of data, and analysis and interpretation of data; drafted the manuscript; and approved the final version for publication. D.Y.: conception and design, acquisition of data, and analysis and interpretation of data; revised the manuscript critically for important intellectual content; and approved the final version for publication. S.K.: conception and design, acquisition of data, and analysis and interpretation of data; revised the manuscript critically for important intellectual content; and approved the final version for publication. K.S.: conception, design, and acquisition of data; revised the manuscript critically for important intellectual content; and approved the final version for publication. Y.I.: conception, design, and acquisition of data; revised the manuscript critically for important intellectual content; and approved the final version for publication. C.Y: conception, design, and acquisition of data; revised the manuscript critically for important intellectual content; and approved the final version for publication. Y.T: conception, design, and acquisition of data; revised the manuscript critically for important intellectual content; and approved the final version for publication. T.N.: conception and design, acquisition of data, and analysis and interpretation of data; revised the manuscript critically for important intellectual content; and approved the final version for publication. M.S.: conception and design, acquisition of data, and analysis and interpretation of data; revised the manuscript critically for important intellectual content; and approved the final version for publication. T.A.: conception and design, acquisition of data, and analysis and interpretation of data; revised the manuscript critically for important intellectual content; and approved the final version for publication. M.T.: conception and design, acquisition of data, and analysis and interpretation of data; revised the manuscript critically for important intellectual content; and approved the final version for publication. H.T.: conception and design, acquisition of data, and analysis and interpretation of data; revised the manuscript critically for important intellectual content; and approved the final version for publication. Y.D: conception and design, acquisition of data, and analysis and interpretation of data; revised the manuscript critically for important intellectual content; and approved the final version for publication. H.E.: conception and design and analysis and interpretation of the data; revised the manuscript critically for important intellectual content; and approved the final version for publication.
Corresponding author
Ethics declarations
Competing interests
S.K. has received honoraria from AstraZeneca, Novartis, Olympus, and Taiho. All other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eguchi, S., Yamada, D., Kobayashi, S. et al. IL-33 released during liver resection facilitates intrahepatic cholangiocarcinoma growth via cytokine secretion in cancer-associated fibroblasts. Br J Cancer 134, 519–529 (2026). https://doi.org/10.1038/s41416-025-03256-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-03256-2