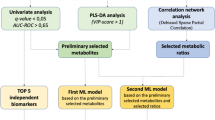
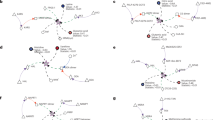
Abstract
Background
Adverse symptoms are the main reason for unexpected hospital admissions in lung cancer patients. The fatigue-pain-sleep disturbance symptom cluster is the core symptom cluster among those undergoing chemotherapy, significantly affecting the clinical outcomes of patients. The mechanisms behind the core symptom cluster haven’t been well understood and may relate to metabolic disorders. Understanding the related mechanisms is crucial for symptom management and patient outcomes.
Methods
One hundred lung cancer patients undergoing chemotherapy were involved in this cross-sectional study. During their third to fifth chemotherapy, they were asked to complete questionnaires that evaluated the condition of fatigue, pain, and sleep disturbance. Patients were categorized into high, moderate, and low core symptom cluster groups using the K-means algorithm. Comparison was conducted on demographic data, symptoms, and symptom interference among groups. Plasma samples from patients were collected for untargeted metabolomics, and the differential metabolites among groups were identified. Pathway enrichment analysis was performed to understand the potential mechanism of the core symptom cluster. Trend analysis was conducted on the differential metabolites to identify the significant clusters of metabolites with similar variation among groups. Receiver Operating Characteristic curves were generated to evaluate the distinguish efficacy of the metabolites panel for the core symptom cluster.
Results
The K-means algorithm categorized 100 patients into three groups based on core symptom cluster, including 46 in low, 35 in moderate, and 19 in high. Most of the cancer-related symptoms and symptom interference were increased progressively with the severity of the core symptom cluster. The metabolomic analysis identified 1,334 annotated metabolites. The high and moderate groups showed a higher similarity in metabolic profile, yet were distinct from the low group. Choline metabolism in cancer was the highest enrichment pathway in the Kyoto Encyclopedia of Genes and Genomes analysis. Trend analysis identified a panel of 24 metabolites that increased with core symptom cluster progression, distinguishing 87.8% of patients with moderate or high severity from those with low.
Conclusions
This study is the first to explore the metabolic profile associated with the core symptom cluster in lung cancer, revealing significant changes in the clinical phenotypes and metabolic disorders in patients with moderate to high core symptom cluster. Dysregulated choline metabolism emerged as a key signature within the broader metabolic disturbances linked to core symptom cluster. We identified a promising panel of metabolites for diagnosing the core symptom cluster, which are expected to serve as biomarkers in the future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
All analyses central to this study were performed with publicly available software packages whose versions are documented in the Methods section. No custom code was generated beyond routine parameter settings within programs.
References
Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. 2024;4:47–53.
Dafni U, Tsourti Z, Vervita K, Peters S. Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first-line treatment for advanced non-small cell lung cancer. A systematic review and network meta-analysis. Lung Cancer. 2019;134:127–40.
Prieto-Callejero B, Rivera F, Fagundo-Rivera J, Romero A, Romero-Martin M, Gomez-Salgado J, et al. Relationship between chemotherapy-induced adverse reactions and health-related quality of life in patients with breast cancer. Medicine (Baltim). 2020;99:e21695.
Lim KE, Kim SR, Kim HK, Kim SR. Symptom Clusters and Quality of Life in Subjects With COPD. Respir Care. 2017;62:1203–11.
Luo Y, Mao D, Zhang L, Yang Z, Miao J, Zhang L. Identification of symptom clusters and sentinel symptoms during the first cycle of chemotherapy in patients with lung cancer. Support Care Cancer. 2024;32:385.
Teng L, Zhou Z, Yang Y, Sun J, Dong Y, Zhu M, et al. Identifying central symptom clusters and correlates in patients with lung cancer post-chemotherapy: A network analysis. Asia Pac J Oncol Nurs. 2024;11:100383.
Zhang G, Weng H, Li Y, Li P, Gong Y, Chen J, et al. Symptom clusters and their predictors in patients with lung cancer and treated with programmed cell death protein 1 immunotherapy. Asia Pac J Oncol Nurs. 2022;9:100103.
Abolfathi H, Sheikhpour M, Shahraeini SS, Khatami S, Nojoumi SA. Studies in lung cancer cytokine proteomics: a review. Expert Rev Proteom. 2021;18:49–64.
Li H, Marsland AL, Conley YP, Sereika SM, Bender CM. Genes involved in the HPA axis and the symptom cluster of fatigue, depressive symptoms, and anxiety in women with breast cancer during 18 months of adjuvant therapy. Biol Res Nurs. 2020;22:277–86.
Huang S, Guo Y, Li ZW, Shui G, Tian H, Li BW, et al. Identification and validation of plasma metabolomic signatures in precancerous gastric lesions that progress to cancer. JAMA Netw Open. 2021;4:e2114186.
Feng LR, Barb JJ, Regan J, Saligan LN. Plasma metabolomic profile associated with fatigue in cancer patients. Cancer Med. 2021;10:1623–33.
Zhu YF, Linher-Melville K, Wu J, Fazzari J, Miladinovic T, Ungard R, et al. Bone cancer-induced pain is associated with glutamate signalling in peripheral sensory neurons. Mol Pain. 2020;16:1744806920911536.
Papachristou N, Barnaghi P, Cooper B, Kober KM, Maguire R, Paul SM, et al. Network analysis of the multidimensional symptom experience of oncology. Sci Rep. 2019;9:2258.
Kwekkeboom KL, Tostrud L, Costanzo E, Coe CL, Serlin RC, Ward SE, et al. The role of inflammation in the pain, fatigue, and sleep disturbance symptom cluster in advanced cancer. J Pain Symptom Manag. 2018;55:1286–95.
Bright HR, Chandy SJ, Chacko RT, Backianathan S. Intercycle unplanned hospital admissions due to cisplatin-based chemotherapy regimen-induced adverse reactions: a retrospective analysis. Curr Drug Saf. 2019;14:182–91.
Denis F, Lethrosne C, Pourel N, Molinier O, Pointreau Y, Domont J, et al. Randomized trial comparing a web-mediated follow-up with routine surveillance in lung cancer patients. J Natl Cancer Inst. 2017;109
Denis F, Basch E, Septans AL, Bennouna J, Urban T, Dueck AC, et al. Two-year survival comparing web-based symptom monitoring vs routine surveillance following treatment for lung cancer. JAMA. 2019;321:306–7.
Pickering CEZ, Winstead V, Yildiz M, Wang D, Yefimova M, Pickering AM. Subsyndromes and symptom clusters: Multilevel factor analysis of behavioral and psychological symptoms of dementia with intensive longitudinal data. Alzheimers Dement. 2024;20:6699–708.
Kee MZL, Cremaschi A, De Iorio M, Chen H, Montreuil T, Nguyen TV, et al. Perinatal trajectories of maternal depressive symptoms in prospective, community-based cohorts across 3 continents. JAMA Netw Open. 2023;6:e2339942.
Epsi NJ, Chenoweth JG, Blair PW, Lindholm DA, Ganesan A, Lalani T, et al. Precision symptom phenotyping identifies early clinical and proteomic predictors of distinct COVID-19 Sequelae[J]. J Infect Dis. 2025;232:39–49.
Xu H, Mohamed M, Flannery M, Peppone L, Ramsdale E, Loh KP, et al. An unsupervised machine learning approach to evaluating the association of symptom clusters with adverse outcomes among older adults with advanced cancer: a secondary analysis of a randomized clinical trial. JAMA Netw Open. 2023;6:e234198.
Luo Y, Zhang L, Mao D, Yang Z, Zhu B, Miao J, et al. Symptom clusters and impact on quality of life in lung cancer patients undergoing chemotherapy. Qual Life Res. 2024;33:3363–75.
Gyamfi J, Kim J, Choi J. Cancer as a metabolic disorder. Int J Mol Sci, 2022,23.
Glunde K, Bhujwalla ZM, Ronen SM. Choline metabolism in malignant transformation. Nat Rev Cancer. 2011;11:835–48.
Liu C, Liu D, Wang F, Liu Y, Xie J, Xie J, et al. Construction of a novel choline metabolism-related signature to predict prognosis, immune landscape, and chemotherapy response in colon adenocarcinoma. Front Immunol. 2022;13:1038927.
Watson GA, Sanz-Garcia E, Zhang WJ, Liu ZA, Yang SC, Wang B, et al. Increase in serum choline levels predicts for improved progression-free survival (PFS) in patients with advanced cancers receiving pembrolizumab. J Immunother Cancer, 2022,10.
Nagy-Szakal D, Barupal DK, Lee B, Che X, Williams BL, Kahn EJR, et al. Insights into myalgic encephalomyelitis/chronic fatigue syndrome phenotypes through comprehensive metabolomics. Sci Rep. 2018;8:10056.
Rowley TJ, McKinstry A, Greenidge E, Smith W, Flood P. Antinociceptive and anti-inflammatory effects of choline in a mouse model of postoperative pain. Br J Anaesth. 2010;105:201–7.
Pak VM, Dai F, Keenan BT, Gooneratne NS, Pack AI. Lower plasma choline levels are associated with sleepiness symptoms. Sleep Med. 2018;44:89–96.
Zhou Z, Yang Y, Sun J, Dong Y, Zhu M, Wang T, et al. Heterogeneity of pain-fatigue-sleep disturbance symptom clusters in lung cancer patients after chemotherapy: a latent profile analysis. Support Care Cancer. 2024;32:821.
Shim EJ, Jeong D, Jung D, Kim TY, Lee KH, Im SA, et al. Do posttraumatic stress symptoms predict trajectories of sleep disturbance and fatigue in patients with breast cancer? A parallel-process latent growth model. Psychooncology. 2022;31:1286–93.
Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–46.
Wang XS, Wang Y, Guo H, Mendoza TR, Hao XS, Cleeland CS. Chinese version of the M. D. Anderson Symptom Inventory: validation and application of symptom measurement in cancer patients. Cancer. 2004;101:1890–901.
Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–96.
Wang XS, Hao XS, Wang Y, Guo H, Jiang YQ, Mendoza TR, et al. Validation study of the Chinese version of the Brief Fatigue Inventory (BFI-C). J Pain Symptom Manage. 2004;27:322–32.
Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23:129–38.
Wang XS, Mendoza TR, Gao SZ, Cleeland CS. The Chinese version of the Brief Pain Inventory (BPI-C): its development and use in a study of cancer pain. Pain. 1996;67:407–16.
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213.
Funding
The work was supported by the National Natural Science Foundation of China (No. 72374097). The funders had no role in study design, data analysis, or decision to publish.
Author information
Authors and Affiliations
Contributions
ZW and LZ designed the study and obtained grant support; YX and YL conducted the experiments; DM, Le Z, RB, ZY, YZ, and FZ recruited the patients and collected samples; YX performed data analysis and wrote the manuscript. All authors saw and approved the final version, and no other person made a substantial contribution to the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This research has been approved by The Research Ethics Board of Nanfang Hospital of Southern Medical University (NFEC-202312-K6), and all patients have signed the informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, Y., Luo, Y., Mao, D. et al. Metabolic dysregulation associated with core symptom cluster in lung cancer patients undergoing chemotherapy. Br J Cancer 134, 295–305 (2026). https://doi.org/10.1038/s41416-025-03262-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-03262-4