Abstract
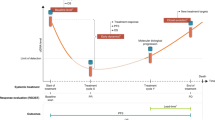
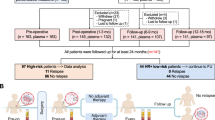
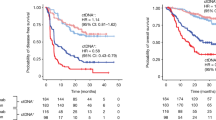
Hepatocellular carcinoma (HCC) relapse remains high after curative-intent treatment due to occult minimal residual disease. Circulating tumour DNA (ctDNA) has emerged as a noninvasive biomarker. Systematic search of MEDLINE, EMBASE and the Cochrane Library up to November 2024 identified studies evaluating plasma ctDNA in non-metastatic HCC patients undergoing curative-intent treatment. Hazard ratios (HRs) and 95% confidence intervals (CIs) for recurrence-free survival (RFS) and overall survival (OS) were pooled using random-effects models; sensitivity and specificity for predicting recurrence were summarised. Ten retrospective studies (n = 793) met inclusion criteria. Postoperative ctDNA positivity was associated with shorter RFS (HR 4.48; 95% CI 2.56–7.82; I² = 78%; p < 0.001) and worse OS (HR 2.99; 95% CI 1.94–4.61; I² = 47%; p < 0.001). Baseline ctDNA detection predicted reduced RFS (HR 3.54; 95% CI 1.97–6.38; I² = 35%; p < 0.001). Sensitivity ranged 33–82% and specificity 41–100%, reflecting methodological heterogeneity. Leave-one-out analyses confirmed robustness. Plasma ctDNA is a potent prognostic marker of recurrence and survival in non-metastatic HCC. Prospective trials incorporating ctDNA could optimise postoperative surveillance and guide adjuvant therapy selection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D. Chapter One - Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. In: Sarkar D, Fisher PB, editors. Advances in Cancer Research [Internet]. Academic Press; 2021 [cited 2025]. p. 1–61. (Mechanisms and Therapy of Liver Cancer; vol. 149). Available from: https://www.sciencedirect.com/science/article/pii/S0065230X20300671
Cucchetti A, Elshaarawy O, Han G, Chong CCN, Serra C, O’Rourke JM, et al. Potentially curative therapies’ for hepatocellular carcinoma: how many patients can actually be cured?. Br J Cancer. 2023;128:1665–71.
Nishida N. Long-term prognosis and management of hepatocellular carcinoma after curative treatment. Clin Mol Hepatol. 2020;26:480.
Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150:835–53.
Abdelhamed W, El-Kassas M. Hepatocellular carcinoma recurrence: Predictors and management. Liver Res. 2023;7:321.
Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–54.
Yopp A, Kudo M, Chen M, Cheng AL, Kaseb AO, Lee HC, et al. LBA39 Updated efficacy and safety data from IMbrave050: Phase III study of adjuvant atezolizumab (atezo) + bevacizumab (bev) vs active surveillance in patients (pts) with resected or ablated high-risk hepatocellular carcinoma (HCC). Ann Oncol. 2024;35:S1230.
Hennedige T, Venkatesh SK. Imaging of hepatocellular carcinoma: diagnosis, staging and treatment monitoring. Cancer Imaging. 2013;12:530.
Wan JCM, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, Caldas C, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17:223–38.
Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24–224ra24.
Moding EJ, Liu Y, Nabet BY, Chabon JJ, Chaudhuri AA, Hui AB, et al. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small-cell lung cancer. Nat Cancer. 2020;1:176–83.
Coombes RC, Page K, Salari R, Hastings RK, Armstrong A, Ahmed S, et al. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res J Am Assoc Cancer Res. 2019;25:4255–63.
Circulating Tumor DNA Analyses as Markers of Recurrence Risk and Benefit of Adjuvant Therapy for Stage III Colon Cancer. Cancer Biomarkers. JAMA Oncology. JAMA Network [Internet]. [cited 2025]. Available from: https://jamanetwork.com/journals/jamaoncology/fullarticle/2752788#google_vignette
Guo B, Chen Q, Liu Z, Chen X, Zhu P. Adjuvant therapy following curative treatments for hepatocellular carcinoma: current dilemmas and prospects. Front Oncol. 2023;13:1098958.
Moris D, Martinino A, Schiltz S, Allen PJ, Barbas A, Sudan D, et al. Advances in the treatment of hepatocellular carcinoma: An overview of the current and evolving therapeutic landscape for clinicians. CA Cancer J Clin [Internet]. [cited 2025]. Available from: https://onlinelibrary.wiley.com/doi/abs/10.3322/caac.70018
Kopystecka A, Patryn R, Leśniewska M, Budzyńska J, Kozioł I. The Use of ctDNA in the diagnosis and monitoring of hepatocellular carcinoma—literature review. Int J Mol Sci. 2023;24:9342.
Yang W, Nguyen R, Safri F, Shiddiky MJA, Warkiani ME, George J, et al. Liquid biopsy in hepatocellular carcinoma: ctdna as a potential biomarker for diagnosis and prognosis.Curr Oncol Rep.2025;27:791–802.
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews | The BMJ [Internet]. [cited 2025]. Available from https://www.bmj.com/content/372/bmj.n71
Assessing bias in studies of prognostic factors - PubMed [Internet]. [cited 2025]. Available from: https://pubmed.ncbi.nlm.nih.gov/23420236/
Chapter 10: Analysing data and undertaking meta-analyses | Cochrane [Internet]. [cited 2025 Aug 30]. Available from https://www.cochrane.org/authors/handbooks-and-manuals/handbook/current/chapter-10
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61:991–6.
Abdelrahim M, Mejia A, Esmail A, Barrera Gutierrez JC, Ouf M, Franses JW, et al. Feasibility of personalized and tumor-informed circulating tumor DNA assay for early recurrence detection in patients with hepatocellular carcinoma. JCO Precis Oncol. 2025;9:e2400934.
Campani C, Imbeaud S, Couchy G, Ziol M, Hirsch TZ, Rebouissou S, et al. Circulating tumour DNA in patients with hepatocellular carcinoma across tumour stages and treatments. Gut. 2024;73:1870–82.
Hu J, Tang H, Jia CC, Zhang XY, Xu Y, Tan JP, et al. Personalized MRD assessment in perisurgical ctDNA for prognostic prediction in hepatocellular carcinoma. Clin Cancer Res J Am Assoc Cancer Res. 2025;31:1047–56.
Pan M, Cai J, Xu Y, Zhong K, Chen T. Abstract 5118: Postoperative minimal residual disease to predict the risk of hepatocellular carcinoma (HCC) recurrence using plasma-only circulating tumor DNA assay. Cancer Res. 2022;82:5118.
Wang J, Huang A, Wang YP, Yin Y, Fu PY, Zhang X, et al. Circulating tumor DNA correlates with microvascular invasion and predicts tumor recurrence of hepatocellular carcinoma. Ann Transl Med. 2020;8:237.
Xu Y, Cai J, Zhong K, Wen Y, Cai L, He G, et al. Plasma-only circulating tumor DNA analysis detects minimal residual disease and predicts early relapse in hepatocellular carcinoma patients undergoing curative resection. Front Oncol. 2023;13:1119744.
Ye K, Fan Q, Yuan M, Wang D, Xiao L, Long G, et al. Prognostic value of postoperative circulating tumor DNA in patients with early- and intermediate-stage hepatocellular carcinoma. Front Oncol. 2022;12:834992.
Zhao L, Jiang L, Liu Y, Wang X, Song J, Sun Y, et al. Integrated analysis of circulating tumour cells and circulating tumour DNA to detect minimal residual disease in hepatocellular carcinoma. Clin Transl Med. 2022;12:e793.
Zhu GQ, Liu WR, Tang Z, Qu WF, Fang Y, Jiang XF, et al. Serial circulating tumor DNA to predict early recurrence in patients with hepatocellular carcinoma: a prospective study. Mol Oncol. 2022;16:549–61.
Huang A, Guo DZ, Zhang X, Sun Y, Zhang SY, Zhang X, et al. Serial circulating tumor DNA profiling predicts tumor recurrence after liver transplantation for liver cancer. Hepatol Int. 2024;18:254–64.
Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology. 2018;154:1706–1718.e1.
Piñero F, Dirchwolf M, Pessôa MG. Biomarkers in hepatocellular carcinoma: diagnosis, prognosis and treatment response assessment. Cells. 2020;9:1370.
Eilard MS, Åberg F. Combination of biomarkers for the detection of hepatocellular carcinoma. Ann Transl Med. 2020;8:1283–1283.
Advancements in the Diagnosis of Hepatocellular Carcinoma [Internet]. [cited 2025]. Available from: https://www.mdpi.com/2673-8937/3/1/5.
Multiparametric liver MRI for predicting early recurrence of hepatocellular carcinoma after microwave ablation. Cancer Imaging. Full Text [Internet]. [cited 2025]. Available from: https://cancerimagingjournal.biomedcentral.com/articles/10.1186/s40644-022-00471-5.
Chartampilas E, Rafailidis V, Georgopoulou V, Kalarakis G, Hatzidakis A, Prassopoulos P. Current imaging diagnosis of hepatocellular carcinoma. Cancers. 2022;14:3997.
Ma L, Guo H, Zhao Y, Liu Z, Wang C, Bu J, et al. Liquid biopsy in cancer: current status, challenges and future prospects. Signal Transduct Target Ther. 2024;9:1–36.
Pandey S, Yadav P. Liquid biopsy in cancer management: integrating diagnostics and clinical applications. Pract Lab Med. 2025;43:e00446.
Bartolomucci A, Nobrega M, Ferrier T, Dickinson K, Kaorey N, Nadeau A, et al. Circulating tumor DNA to monitor treatment response in solid tumors and advance precision oncology. Npj Precis Oncol. 2025;9:1–19.
Faulkner LG, Howells LM, Pepper C, Shaw JA, Thomas AL. The utility of ctDNA in detecting minimal residual disease following curative surgery in colorectal cancer: a systematic review and meta-analysis. Br J Cancer. 2023;128:297–309.
Galli E, Patelli G, Villa F, Gri N, Mazzarelli C, Mangoni I, et al. Circulating blood biomarkers for minimal residual disease in hepatocellular carcinoma: a systematic review. Cancer Treat Rev. 2025;135:102908.
Kornasiewicz O. A prospective randomized trial assessing the impact of ctDNA testing in patients after liver resection or transplantation due to metastases from colorectal cancer or hepatocellular carcinoma (HCC) on treatment strategies and long-term survival" [Internet]. clinicaltrials.gov; 2025 May [cited 2025. Report No.: NCT07001085. Available from: https://clinicaltrials.gov/study/NCT07001085.
Zhujiang Hospital. Compare the Accuracy of Circulating Tumor DNA Longitudinal Monitoring Minimal Residual Disease and Microvascular Invasion Result in Predicting Postoperative Recurrence of Hepatocellular Carcinoma [Internet]. clinicaltrials.gov; 2024 June [cited 2025. Report No.: NCT06449846. Available from: https://clinicaltrials.gov/study/NCT06449846.
Singlera Genomics Inc. Clinical Research on Dynamic Monitoring MRD Via Plasma ctDNA Predicting Postoperative Recurrence and Progression After Systemic Therapy of Hepatocellular Carcinoma [Internet]. clinicaltrials.gov; 2024 Jan [cited 2025 Aug 30]. Report No.: NCT06178809. Available from: https://clinicaltrials.gov/study/NCT06178809.
Henriksen TV, Reinert T, Christensen E, Sethi H, Birkenkamp-Demtröder K, Gögenur M, et al. The effect of surgical trauma on circulating free DNA levels in cancer patients-implications for studies of circulating tumor DNA. Mol Oncol. 2020;14:1670–9.
Henriksen TV, Demuth C, Frydendahl A, Nors J, Nesic M, Rasmussen MH, et al. Timing of ctDNA analysis aimed at guiding adjuvant treatment in colorectal cancer. Clin Cancer Res J Am Assoc Cancer Res. 2025;31:1676–85.
Dang DK, Park BH. Circulating tumor DNA: current challenges for clinical utility. J Clin Invest. 2022;132:e154941.
A comprehensive overview of minimal residual disease in the management of early-stage and locally advanced non-small cell lung cancer | npj Precision Oncology [Internet]. [cited 2025 July 6]. Available from: https://www.nature.com/articles/s41698-025-00984-9?utm_source=chatgpt.com.
Merker JD, Oxnard GR, Compton C, Diehn M, Hurley P, Lazar AJ, et al. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J Clin Oncol J Am Soc Clin Oncol. 2018;36:1631–41.
Keller L, Belloum Y, Wikman H, Pantel K. Clinical relevance of blood-based ctDNA analysis: mutation detection and beyond. Br J Cancer. 2021;124:345–58.
Current status of ctDNA in precision oncology for hepatocellular carcinoma. J Exp Clin Cancer Res. Full Text [Internet]. [cited 2025 Aug 30]. Available from: https://jeccr.biomedcentral.com/articles/10.1186/s13046-021-01940-8.
Husain H, Pavlick DC, Fendler BJ, Madison RW, Decker B, Gjoerup O, et al. Tumor fraction correlates with detection of actionable variants across > 23,000 circulating tumor DNA samples. JCO Precis Oncol. 2022;6:e2200261.
Author information
Authors and Affiliations
Contributions
Isabella Buonopane and Erick F. Saldanha conceptualised the study and coordinated the systematic review process. Júnior Samuel Alonso de Menezes and Lucas Diniz da Conceição performed the literature search and data extraction. Camila Mariana de Paiva Reis and Luís F. Leite da Silva contributed to quality assessment and data analysis. Thiago Francischetto assisted in data interpretation and drafting of the manuscript. Renata D’Alpino Peixoto and Tiago Biachi de Castria provided critical revisions and contributed to the final approval of the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study is a systematic review and meta-analysis of previously published data. No new data involving human participants, human tissue, or personally identifiable information were collected or analysed. Therefore, ethical approval and informed consent were not required. All included studies had obtained prior approval from their respective institutional ethics committees. This meta-analysis was conducted in accordance with the principles of the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Buonopane, I.R., Saldanha, E.F., de Menezes, J.S.A. et al. Circulating tumour DNA for a minimal residual disease assessment and recurrence risk in hepatocellular carcinoma: a systematic review and meta-analysis. Br J Cancer (2025). https://doi.org/10.1038/s41416-025-03296-8
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41416-025-03296-8