Abstract
Background
BRCA1 and BRCA2 are tumor suppressor genes essential for DNA repair. Mutations in these genes significantly increase breast (BC) and ovarian cancer (OC) risk, with BRCA1-positive facing a 70% BC and 40% OC lifetime risk. While guidelines for BRCA-positive are well established, recommendations for BC surveillance in BRCA-patients already diagnosed with OC remain limited. This meta-analysis evaluates BC risk post-OC in BRCA-mutated women.
Methods
A systematic search of PubMed, Embase, and the Cochrane Library was performed. Single-arm outcomes were pooled using meta-analysis of proportions, and survival data were synthesized using hazard ratio (HR), both with 95% confidence intervals (CIs). Heterogeneity was assessed using the I² statistic. All analyses were conducted in R (version 4.3.2).
Results
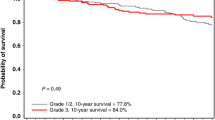
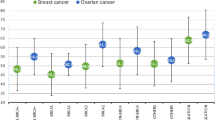
A total of 2380 patients from 10 retrospective cohort studies were included. Among them, 181 (8%; 95% CI: 6%–11%) developed BC post-OC, with similar rates observed for BRCA1 and BRCA2-mutated (9%; 95% CI: 7%–12%). In overall survival analysis, BRCA-mutated patients who developed BC after OC had significantly improved outcomes compared to those with OC only (HR = 0.4657; P < 0.001).
Conclusion
This meta-analysis underscores the need for tailored BC surveillance and evidence-based screening guidelines in BRCA-mutated OC survivors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
More data can be accessed upon request to the corresponding author. Pedro Henrique de Souza Wagner had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
Albertsen H, Plaetke R, Ballard L, Fujimoto E, Connolly J, Lawrence E, et al. Genetic mapping of the BRCA1 region on chromosome 17q21. Am J Hum Genet. 1994;54:516–25.
Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–92.
Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. 2011;12:68–78.
Dubsky P, Jackisch C, Im SA, Hunt KK, Li CF, Unger S, et al. BRCA genetic testing and counseling in breast cancer: how do we meet our patients’ needs?. NPJ Breast Cancer. 2024;10:77.
Antoniou A, Pharoah PDP, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–30.
Lubinski J, Huzarski T, Byrski T, Lynch HT, Cybulski C, Ghadirian P, et al. The risk of breast cancer in women with a BRCA1 mutation from North America and Poland. Int J Cancer J Int Cancer. 2012;131:229–34.
Daly MB, Pilarski R, Yurgelun MB, Berry MP, Buys SS, Dickson P, et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 1.2020. J Natl Compr Cancer Netw JNCCN. 2020;18:380–91.
Domchek SM, Jhaveri K, Patil S, Stopfer JE, Hudis C, Powers J, et al. Risk of metachronous breast cancer after BRCA mutation-associated ovarian cancer. Cancer. 2013;119:1344–8.
Gangi A, Cass I, Paik D, Barmparas G, Karlan B, Dang C, et al. Breast cancer following ovarian cancer in BRCA mutation carriers. JAMA Surg. 2014;149:1306–13.
Fong A, Cass I, John C, Gillen J, Moore KM, Gangi A, et al. Breast cancer surveillance following ovarian cancer in BRCA mutation carriers. Am Surg. 2020;86:1243–7.
Moraes FCA, de, Moro LD, Souza MEC, de Rodrigues ALSO, Sano VKT, Barbosa BF, et al. Prevalence of cardiometabolic outcomes in women who underwent salpingo-oophorectomy to prevent hereditary breast and ovarian cancer: a meta-analysis. Fam Cancer. 2024;24:5.
Wong SM, Apostolova C, Eisenberg E, Foulkes WD. Counselling framework for germline BRCA1/2 and PALB2 carriers considering risk-reducing mastectomy. Curr Oncol Tor Ont. 2024;31:350–65.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
Furuya-Kanamori L, Barendregt JJ, Doi SAR. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid Based Healthc. 2018;16:195–203.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–60.
von Hippel PT. The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med Res Methodol. 2015;15:35.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Page MJ, Higgins JP, Sterne JA. Assessing risk of bias due to missing results in a synthesis. In: Cochrane Handbook for Systematic Reviews of Interventions [Internet]. John Wiley & Sons, Ltd, 2019 [cited 2025 Oct 9]. p. 349–74. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119536604.ch13.
Vencken PMLH, Kriege M, Hooning M, Menke-Pluymers MB, Heemskerk-Gerritsen BAM, van Doorn LC, et al. The risk of primary and contralateral breast cancer after ovarian cancer in BRCA1/BRCA2 mutation carriers: implications for counseling. Cancer. 2013;119:955–62.
McGee J, Giannakeas V, Karlan B, Lubinski J, Gronwald J, Rosen B, et al. Risk of breast cancer after a diagnosis of ovarian cancer in BRCA mutation carriers: Is preventive mastectomy warranted? Gynecol Oncol. 2017;145:346–51.
Artioli G, Giannone G, Valabrega G, Maggiorotto F, Genta S, Pignata S, et al. Characteristics and outcome of BRCA mutated epithelial ovarian cancer patients in Italy: a retrospective multicenter study (MITO 21). Gynecol Oncol. 2021;161:755–61.
Safra T, Waissengrin B, Gerber D, Bernstein-Molho R, Klorin G, Salman L, et al. Breast cancer incidence in BRCA mutation carriers with ovarian cancer: a longitudal observational study. Gynecol Oncol. 2021;162:715–9.
John CS, Fong A, Alban R, Gillen J, Moore KM, Walsh CS, et al. Breast cancer surveillance following ovarian cancer in BRCA mutation carriers. Gynecol Oncol. 2022;164:202–7.
Nañez A, Stram DA, Bethan Powell C, Garcia C. Breast cancer risk in BRCA mutation carriers after diagnosis of epithelial ovarian cancer is lower than in carriers without ovarian cancer. Gynecol Oncol Rep. 2022;39:100899.
Oliveira D, Fernandes S, Miguel I, Fragoso S, Vaz F. Is there a role for risk-reducing bilateral breast surgery in BRCA1/2 ovarian cancer survivors? an observational study. Curr Oncol Tor Ont. 2023;30:7810–7.
Mavaddat N, Peock S, Frost D, Ellis S, Platte R, Fineberg E, et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013;105:812–22.
Godet I, Gilkes DM. BRCA1 and BRCA2 mutations and treatment strategies for breast cancer. Integr Cancer Sci Ther. 2017;4:1.
Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402–16.
Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol. 2010;28:375–9.
Turner NC, Tutt ANJ. Platinum chemotherapy for BRCA1-related breast cancer: do we need more evidence?. Breast Cancer Res BCR. 2012;14:115.
Barber LJ, Sandhu S, Chen L, Campbell J, Kozarewa I, Fenwick K, et al. Secondary mutations in BRCA2 associated with clinical resistance to a PARP inhibitor. J Pathol. 2013;229:422–9.
Lord CJ, Tutt ANJ, Ashworth A. Synthetic lethality and cancer therapy: lessons learned from the development of PARP inhibitors. Annu Rev Med. 2015;66:455–70.
González-Martín A, Pothuri B, Vergote I, Christensen RD, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391–402.
Banerjee S, Gonzalez-Martin A, Harter P, Lorusso D, Moore KN, Oaknin A, et al. First-line PARP inhibitors in ovarian cancer: summary of an ESMO Open - Cancer Horizons round-table discussion. ESMO Open. 2020;5:e001110.
Petruchelli N, Daly MB, Pal T. BRCA1- and BRCA2-Associated Hereditary Breast and Ovarian Cancer. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993 [cited 2025 Oct 9]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1247/.
PDQ Cancer Genetics Editorial Board. BRCA1 and BRCA2: Cancer Risks and Management (PDQ®): Health Professional Version. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002 [cited 2025 Oct 9]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK589498/
Greenwood HI, Dodelzon K. Screening in women with BRCA mutations revisited. J Breast Imaging. 2024;6:4–13.
Mainor CB, Isaacs C. Risk Management for BRCA1/BRCA2 mutation carriers without and with breast cancer. Curr Breast Cancer Rep. 2020;12:66–74.
Turner NC, Reis-Filho JS. Basal-like breast cancer and the BRCA1 phenotype. Oncogene. 2006;25:5846–53.
Bayraktar S, Glück S. Systemic therapy options in BRCA mutation-associated breast cancer. Breast Cancer Res Treat. 2012;135:355–66.
Brekelmans CTM, Tilanus-Linthorst MMA, Seynaeve C, vd Ouweland A, Menke-Pluymers MBE, Bartels CCM, et al. Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, BRCA1- and non-BRCA1/2 families as compared to sporadic breast cancer cases. Eur J Cancer Oxf Engl 1990. 2007;43:867–76.
Goodwin PJ, Phillips KA, West DW. Prognosis of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med. 2007;357:1555.
Atchley DP, Albarracin CT, Lopez A, Valero V, Amos CI, Gonzalez-Angulo AM, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol. 2008;26:4282–8.
Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48.
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70.
Tung NM, Garber JE. BRCA1/2 testing: therapeutic implications for breast cancer management. Br J Cancer. 2018;119:141–52.
Funding
Design and conduct of the study: No funding. Collection, management, analysis, and interpretation of the data: No funding. Preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication: No funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. [FCAM] conceived the project; material preparation was performed by [PHSW and GTFUM]. Data collection and analysis were performed by [PHSW, GTFUM, and DMR]. The figures and tables were created by [PHSW]. The first draft of the manuscript was written by [PHSW, GTFUM, DMR, MCFM, and FCAM], and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests that could have influenced the work reported in this manuscript. All authors have reviewed and approved the final version of the manuscript and agree with its submission.
Ethics approval and consent to participate
This study is a systematic review and meta-analysis. All data were obtained exclusively from previously published studies. All methods were performed in accordance with relevant guidelines and regulations. Ethics approval and informed consent were not required, as no new data were collected from human participants or animals. All included studies reported having obtained approval from their respective ethics committees and informed consent from their participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Souza Wagner, P.H., Matheus, G.T.F.U., Monteiro Ribeiro, D. et al. Breast cancer incidence and subtype patterns among BRCA-mutated ovarian cancer patients: a systematic review and meta-analysis. Br J Cancer (2025). https://doi.org/10.1038/s41416-025-03320-x
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41416-025-03320-x