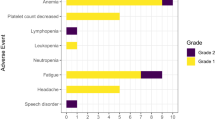
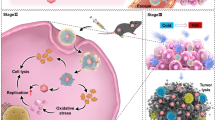
Abstract
Glioblastoma (GBM) represents the most aggressive primary brain tumor, and urgently requires effective treatments. Oncolytic adenovirus (OA) shows promise as a potential candidate for clinical antitumor therapy, including in the treatment of GBM. Nevertheless, the systemic delivery of OA continues to face challenges, leading to significantly compromised antitumor efficacy. In this study, we developed an innovative approach by encapsulating CXCL11-armed OA with tannic acid and Fe3+ (TA-Fe3+) to realize the systemic delivery of OA. The nanocarrier’s ability to protect the OA from elimination by host immune response was evaluated in vitro and in vivo. We evaluated the antitumor effect and safety profile of OA@TA-Fe3+ in a GBM-bearing mice model. OA@TA-Fe3+ effectively safeguarded the virus from host immune clearance and extended its circulation in vivo. After targeting tumor sites, TA-Fe3+ could dissolve and release Fe3+ and OA. Fe3+-induced O2 production from H2O2 relieved the hypoxic state, and promoted OA replication, leading to a remarkable alteration of tumor immune microenvironment and enhancement in antitumor efficacy. Moreover, the systemic delivery of OA@TA-Fe3+ was safe without inflammation or organ damage. Our findings demonstrated the promising potential of systemically delivering the engineered OA for effective oncolytic virotherapy against GBM.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data generated and analyzed during this study are available on request.
References
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96.
Tan AC, Ashley DM, López GY, Malinzak M, Friedman HS, Khasraw M. Management of glioblastoma: state of the art and future directions. CA Cancer J Clin. 2020;70:299–312.
Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2016;15:660.
Ghajar-Rahimi G, Kang K-D, Totsch SK, Gary S, Rocco A, Blitz S, et al. Clinical advances in oncolytic virotherapy for pediatric brain tumors. Pharmacol Ther. 2022;239:108193.
Yuan B, Wang G, Tang X, Tong A, Zhou L. Immunotherapy of glioblastoma: recent advances and future prospects. Hum Vaccin Immunother. 2022;18:2055417.
Harrington K, Freeman DJ, Kelly B, Harper J, Soria J-C. Optimizing oncolytic virotherapy in cancer treatment. Nat Rev Drug Discov. 2019;18:689–706.
Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19:329–36.
Wang G, Zhang Z, Zhong K, Wang Z, Yang N, Tang X, et al. CXCL11-armed oncolytic adenoviruses enhance CAR-T cell therapeutic efficacy and reprogram tumor microenvironment in glioblastoma. Mol Ther. 2023;31:134–53.
Thambi T, Hong J, Yoon A-R, Yun C-O. Challenges and progress toward tumor-targeted therapy by systemic delivery of polymer-complexed oncolytic adenoviruses. Cancer Gene Ther. 2022;29:1321–31.
Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, et al. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–85.
Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, et al. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555–61.
Ferguson MS, Lemoine NR, Wang Y. Systemic delivery of oncolytic viruses: hopes and hurdles. Adv Virol. 2012;2012:805629.
Nemunaitis J, Cunningham C, Buchanan A, Blackburn A, Edelman G, Maples P, et al. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Ther. 2001;8:746–59.
Reid T, Galanis E, Abbruzzese J, Sze D, Andrews J, Romel L, et al. Intra-arterial administration of a replication-selective adenovirus (dl1520) in patients with colorectal carcinoma metastatic to the liver: a phase I trial. Gene Ther. 2001;8:1618–26.
Small EJ, Carducci MA, Burke JM, Rodriguez R, Fong L, van Ummersen L, et al. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol Ther. 2006;14:107–17.
Reid TR, Freeman S, Post L, McCormick F, Sze DY. Effects of Onyx-015 among metastatic colorectal cancer patients that have failed prior treatment with 5-FU/leucovorin. Cancer Gene Ther. 2005;12:673–81.
Reid T, Galanis E, Abbruzzese J, Sze D, Wein LM, Andrews J, et al. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer Res. 2002;62:6070–9.
Martinez-Quintanilla J, Seah I, Chua M, Shah K. Oncolytic viruses: overcoming translational challenges. J Clin Invest. 2019;129:1407–18.
Wong HH, Lemoine NR, Wang Y. Oncolytic viruses for cancer therapy: overcoming the obstacles. Viruses. 2010;2:78–106.
Vassilaki N, Frakolaki E. Virus-host interactions under hypoxia. Microbes Infect. 2017;19:193–203.
Shen BH, Hermiston TW. Effect of hypoxia on Ad5 infection, transgene expression and replication. Gene Ther. 2005;12:902–10.
Schmid M, Ernst P, Honegger A, Suomalainen M, Zimmermann M, Braun L, et al. Adenoviral vector with shield and adapter increases tumor specificity and escapes liver and immune control. Nat Commun. 2018;9:450.
Mu M, Chen H, Fan R, Wang Y, Tang X, Mei L, et al. A tumor-specific ferric-coordinated epigallocatechin-3-gallate cascade nanoreactor for glioblastoma therapy. J Adv Res. 2021;34:29–41.
Wang X, Sun C, Li P, Wu T, Zhou H, Yang D, et al. Vaccine engineering with dual-functional mineral shell: a promising strategy to overcome preexisting immunity. Adv Mater. 2016;28:694–700.
Chen J, Gao P, Yuan S, Li R, Ni A, Chu L, et al. Oncolytic adenovirus complexes coated with lipids and calcium phosphate for cancer gene therapy. ACS Nano. 2016;10:11548–60.
Mu M, Liang X, Zhao N, Chuan D, Chen B, Zhao S, et al. Boosting ferroptosis and microtubule inhibition for antitumor therapy via a carrier-free supermolecule nanoreactor. J Pharm Anal. 2023;13:99–109.
Xue C-C, Li M-H, Zhao Y, Zhou J, Hu Y, Cai K-Y, et al. Tumor microenvironment-activatable Fe-doxorubicin preloaded amorphous CaCO(3) nanoformulation triggers ferroptosis in target tumor cells. Sci Adv. 2020;6:eaax1346.
Zhang L, Wan S-S, Li C-X, Xu L, Cheng H, Zhang X-Z. An adenosine triphosphate-responsive autocatalytic fenton nanoparticle for tumor ablation with self-supplied H(2)O(2) and acceleration of Fe(III)/Fe(II) conversion. Nano Lett. 2018;18:7609–18.
Chen J, Chen F, Zhang L, Yang Z, Deng T, Zhao Y, et al. Self-assembling porphyrins as a single therapeutic agent for synergistic cancer therapy: a one stone three birds strategy. ACS Appl Mater Interfaces. 2021;13:27856–67.
Pan C, Ou M, Cheng Q, Zhou Y, Yu Y, Li Z, et al. Z-scheme heterojunction functionalized pyrite nanosheets for modulating tumor microenvironment and strengthening photo/chemodynamic therapeutic effects. Adv Funct Mater. 2020;30:1906466.
Ge X, Pan M-H, Wang L, Li W, Jiang C, He J, et al. Hypoxia-mediated mitochondria apoptosis inhibition induces temozolomide treatment resistance through miR-26a/Bad/Bax axis. Cell Death Dis. 2018;9:1128.
Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat Rev Cancer. 2014;14:559–67.
Guo ZS, Liu Z, Bartlett DL. Oncolytic immunotherapy: dying the right way is a key to eliciting potent antitumor immunity. Front Oncol. 2014;4:74.
Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111.
Fang Y-Z, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872–9.
Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–91.
Liu C, Liu B, Zhao J, Di Z, Chen D, Gu Z, et al. Nd(3+)-sensitized upconversion metal-organic frameworks for mitochondria-targeted amplified photodynamic therapy. Angew Chem Int Ed Engl. 2020;59:2634–8.
Lin L-S, Huang T, Song J, Ou X-Y, Wang Z, Deng H, et al. Synthesis of copper peroxide nanodots for H(2)O(2) self-supplying chemodynamic therapy. J Am Chem Soc. 2019;141:9937–45.
Yang B, Chen Y, Shi J. Reactive oxygen species (ROS)-based nanomedicine. Chem Rev. 2019;119:4881–985.
Ranji-Burachaloo H, Gurr PA, Dunstan DE, Qiao GG. Cancer treatment through nanoparticle-facilitated fenton reaction. ACS Nano. 2018;12:11819–37.
Tang Z, Liu Y, He M, Bu W. Chemodynamic therapy: tumour microenvironment-mediated Fenton and Fenton-like reactions. Angew Chem Int Ed Engl. 2019;58:946–56.
Liu C, Cao Y, Cheng Y, Wang D, Xu T, Su L, et al. An open source and reduce expenditure ROS generation strategy for chemodynamic/photodynamic synergistic therapy. Nat Commun. 2020;11:1735.
Hamilton NHR, Mahalingam S, Banyer JL, Ramshaw IA, Thomson SA. A recombinant vaccinia virus encoding the interferon-inducible T-cell alpha chemoattractant is attenuated in vivo. Scand J Immunol. 2004;59:246–54.
Moon EK, Wang L-CS, Bekdache K, Lynn RC, Lo A, Thorne SH, et al. Intra-tumoral delivery of CXCL11 via a vaccinia virus, but not by modified T cells, enhances the efficacy of adoptive T cell therapy and vaccines. Oncoimmunology. 2018;7:e1395997.
Hensbergen PJ, Wijnands PGJTB, Schreurs MWJ, Scheper RJ, Willemze R, Tensen CP. The CXCR3 targeting chemokine CXCL11 has potent antitumor activity in vivo involving attraction of CD8+ T lymphocytes but not inhibition of angiogenesis. J Immunother. 2005;28:343–51.
Ménard S, Tomasic G, Casalini P, Balsari A, Pilotti S, Cascinelli N, et al. Lymphoid infiltration as a prognostic variable for early-onset breast carcinomas. Clin Cancer Res. 1997;3:817–9.
Zhu X, Li C, Lu Y, Liu Y, Wan D, Zhu D, et al. Tumor microenvironment-activated therapeutic peptide-conjugated prodrug nanoparticles for enhanced tumor penetration and local T cell activation in the tumor microenvironment. Acta Biomater. 2021;119:337–48.
Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–43.
Yokoda R, Nagalo BM, Vernon B, Oklu R, Albadawi H, DeLeon TT, et al. Oncolytic virus delivery: from nano-pharmacodynamics to enhanced oncolytic effect. Oncolytic Virother. 2017;6:39–49.
Huang L-L, Li X, Zhang J, Zhao QR, Zhang MJ, Liu A-A, et al. MnCaCs-biomineralized oncolytic virus for bimodal imaging-guided and synergistically enhanced anticancer therapy. Nano Lett. 2019;19:8002–9.
Lv P, Liu X, Chen X, Liu C, Zhang Y, Chu C, et al. Genetically engineered cell membrane nanovesicles for oncolytic adenovirus delivery: a versatile platform for cancer virotherapy. Nano Lett. 2019;19:2993–3001.
Hong J, Yun C-O. Overcoming the limitations of locally administered oncolytic virotherapy. BMC Biomed Eng. 2019;1:17.
Lee C-H, Kasala D, Na Y, Lee MS, Kim SW, Jeong JH, et al. Enhanced therapeutic efficacy of an adenovirus-PEI-bile-acid complex in tumors with low coxsackie and adenovirus receptor expression. Biomaterials. 2014;35:5505–16.
Xia M, Luo D, Dong J, Zheng M, Meng G, Wu J, et al. Graphene oxide arms oncolytic measles virus for improved effectiveness of cancer therapy. J Exp Clin Cancer Res. 2019;38:408.
Nosaki K, Hamada K, Takashima Y, Sagara M, Matsumura Y, Miyamoto S, et al. A novel, polymer-coated oncolytic measles virus overcomes immune suppression and induces robust antitumor activity. Mol Ther oncolytics. 2016;3:16022.
Walker S, Busatto S, Pham A, Tian M, Suh A, Carson K, et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics. 2019;9:8001–17.
Evgin L, Kottke T, Tonne J, Thompson J, Huff AL, van Vloten J, et al. Oncolytic virus-mediated expansion of dual-specific CAR T cells improves efficacy against solid tumors in mice. Sci Transl Med. 2022;14:eabn2231.
Acknowledgements
We thank our colleagues for stimulating discussions.
Funding
We declare that this work was supported by funding from the National Natural Science Foundation of China (82073404), 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21003), National Key Research and Development Program of China (2023YFC3403303 and 2023YFC3403304), the Frontiers Medical Center, Tianfu Jincheng Laboratory Foundation (TFJC2023010006) and the Postdoctor Research Fund of West China Hospital, Sichuan University (2024HXBH132).
Author information
Authors and Affiliations
Contributions
GW, MM, and ZZ designed and conducted the experiments and wrote the paper. YC, KZ, YL, and NY performed the data analysis. FL, GG, and AT designed the experiments and supervised the research. All authors discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All animal’s experiments have been approved by the Biomedical Ethics Committee of West China Hospital (2018-061). All methods were performed in accordance with the relevant guidelines and regulations. All PBMC samples from healthy donors as well as tumor samples obtained from GBM patients were approved by Sichuan University West China Hospital Biomedical Ethics Committee. All healthy donors and GBM patients provided written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, G., Mu, M., Zhang, Z. et al. Systemic delivery of tannic acid-ferric-masked oncolytic adenovirus reprograms tumor microenvironment for improved therapeutic efficacy in glioblastoma. Cancer Gene Ther 31, 1804–1817 (2024). https://doi.org/10.1038/s41417-024-00839-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41417-024-00839-8