Abstract
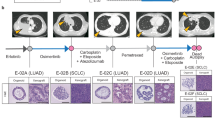
Despite recent advances in treatment strategy, lung cancer remains the leading cause of cancer-related mortality worldwide, and it is a serious threat to human health. Lung adenocarcinoma (LUAD) is the most common histological type of lung cancer, and approximately 40–50% of patients with LUAD in Asian populations have epidermal growth factor receptor (EGFR) mutations. The use of EGFR tyrosine kinase inhibitors (EGFR-TKIs) has revolutionarily improved the prognosis of patients with EGFR-mutated LUAD. However, acquired drug resistance is the main cause of treatment failure. Therefore, new therapeutic strategies are necessary to address the resistance to EGFR-TKIs in patients with LUAD. Cancer stemness-related factors lead to multiple-drug resistance in cancer treatment, including EGFR–TKI resistance. Coiled-coil domain-containing 34 (CCDC34) serves as an oncogene in several types of cancer. However, the role and molecular mechanism of CCDC34 in the malignant progression of LUAD have not been reported to date. In the present study, we found that CCDC34 may be associated with LUAD stemness through weighted gene co-expression network analysis (WGCNA). Furthermore, we demonstrated that CCDC34 promoted LUAD stemness properties through β-catenin-mediated regulation of ATG5-induced autophagy, which was conducive to acquired EGFR-TKI resistance in LUAD in vitro and in vivo. Knockdown CCDC34 can synergistically inhibit tumor growth when combined with EGFR-TKIs. This study reveals a positive association between CCDC34 and the stemness phenotype of LUAD, providing new insights into overcoming EGFR-TKI resistance in LUAD by inhibiting CCDC34 expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data are available upon request made to the first author (yuepingsuda@163.com).
Code availability
(As the reviewer 3 suggested, we have withdrawn the source code of WGCNA from the supplementary material. We apologize for not removing this part from the article).
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
Pennell NA, Neal JW, Chaft JE, Azzoli CG, Jänne PA, Govindan R, et al. SELECT: A Phase II Trial of Adjuvant Erlotinib in Patients With Resected Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer. J Clin Oncol. 2019;37:97–104.
Shen J, Liu G, Qi H, Xiang X, Shao J. JMJD5 inhibits lung cancer progression by facilitating EGFR proteasomal degradation. Cell Death Dis. 2023;14:657.
Passaro A, Jänne PA, Mok T, Peters S. Overcoming therapy resistance in EGFR-mutant lung cancer. Nat Cancer. 2021;2:377–91.
Pan Z, Wang K, Wang X, Jia Z, Yang Y, Duan Y, et al. Cholesterol promotes EGFR-TKIs resistance in NSCLC by inducing EGFR/Src/Erk/SP1 signaling-mediated ERRα re-expression. Mol Cancer. 2022;21:77.
Alam SK, Zhang Y, Wang L, Zhu Z, Hernandez CE, Zhou Y, et al. DARPP-32 promotes ERBB3-mediated resistance to molecular targeted therapy in EGFR-mutated lung adenocarcinoma. Oncogene. 2022;41:83–98.
Song H, Liu D, Wang L, Liu K, Chen C, Wang L, et al. Methyltransferase like 7B is a potential therapeutic target for reversing EGFR-TKIs resistance in lung adenocarcinoma. Mol Cancer. 2022;21:43.
Wu L, Ke L, Zhang Z, Yu J, Meng X. Development of EGFR TKIs and Options to Manage Resistance of Third-Generation EGFR TKI Osimertinib: Conventional Ways and Immune Checkpoint Inhibitors. Front Oncol. 2020;10:602762.
Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46.
Nagaraju GP, Farran B, Luong T, El-Rayes BF. Understanding the molecular mechanisms that regulate pancreatic cancer stem cell formation, stemness and chemoresistance: A brief overview. Semin Cancer Biol. 2023;88:67–80.
Zheng Y, Wang L, Yin L, Yao Z, Tong R, Xue J, et al. Lung Cancer Stem Cell Markers as Therapeutic Targets: An Update on Signaling Pathways and Therapies. Front Oncol. 2022;12:873994.
Heng WS, Gosens R, Kruyt F. Lung cancer stem cells: origin, features, maintenance mechanisms and therapeutic targeting. Biochem Pharm. 2019;160:121–33.
He Y, Jiang X, Duan L, Xiong Q, Yuan Y, Liu P, et al. LncRNA PKMYT1AR promotes cancer stem cell maintenance in non-small cell lung cancer via activating Wnt signaling pathway. Mol Cancer. 2021;20:156.
Sneha S, Nagare RP, Sidhanth C, Krishnapriya S, Garg M, Ramachandran B, et al. The hedgehog pathway regulates cancer stem cells in serous adenocarcinoma of the ovary. Cell Oncol (Dordr). 2020;43:601–16.
Li H, Feng Z, He ML. Lipid metabolism alteration contributes to and maintains the properties of cancer stem cells. Theranostics. 2020;10:7053–69.
Jin ML, Jeong KW. Histone modifications in drug-resistant cancers: From a cancer stem cell and immune evasion perspective. Exp Mol Med. 2023;55:1333–47.
Yi Y, Li P, Huang Y, Chen D, Fan S, Wang J, et al. P21-activated kinase 2-mediated β-catenin signaling promotes cancer stemness and osimertinib resistance in EGFR-mutant non-small-cell lung cancer. Oncogene. 2022;41:4318–29.
Yan R, Fan X, Xiao Z, Liu H, Huang X, Liu J, et al. Inhibition of DCLK1 sensitizes resistant lung adenocarcinomas to EGFR-TKI through suppression of Wnt/β-Catenin activity and cancer stemness. Cancer Lett. 2022;531:83–97.
Jia Z, Zhang Y, Yan A, Wang M, Han Q, Wang K, et al. 1,25-dihydroxyvitamin D3 signaling-induced decreases in IRX4 inhibits NANOG-mediated cancer stem-like properties and gefitinib resistance in NSCLC cells. Cell Death Dis. 2020;11:670.
Jia Z, Wang K, Duan Y, Hu K, Zhang Y, Wang M, et al. Claudin1 decrease induced by 1,25-dihydroxy-vitamin D3 potentiates gefitinib resistance therapy through inhibiting AKT activation-mediated cancer stem-like properties in NSCLC cells. Cell Death Discov. 2022;8:122.
Lei HM, Zhang KR, Wang CH, Wang Y, Zhuang GL, Lu LM, et al. Aldehyde dehydrogenase 1A1 confers erlotinib resistance via facilitating the reactive oxygen species-reactive carbonyl species metabolic pathway in lung adenocarcinomas. Theranostics. 2019;9:7122–39.
Arasada RR, Shilo K, Yamada T, Zhang J, Yano S, Ghanem R, et al. Notch3-dependent β-catenin signaling mediates EGFR TKI drug persistence in EGFR mutant NSCLC. Nat Commun. 2018;9:3198.
Liu Z, Yan W, Liu S, Liu Z, Xu P, Fang W. Regulatory network and targeted interventions for CCDC family in tumor pathogenesis. Cancer Lett. 2023;565:216225.
Wang J, Zhang YW, Zhang NJ, Yin S, Ruan DJ, He N, et al. Coiled-Coil Domain Containing 80 Suppresses Nonylphenol-Induced Colorectal Cancer Cell Proliferation by Inhibiting the Activation of ERK1/2. Front Cell Dev Biol. 2021;9:759820.
Hong WF, Zhu DX, Chen YJ, Shen XZ, Cui YH, Du SS, et al. Coiled-coil domain-containing 154 promotes colorectal cancer proliferation and metastasis via interacting with minichromosome maintenance complex component 2. Cancer Lett. 2023;578:216460.
Qi W, Shao F, Huang Q. Expression of Coiled-Coil Domain Containing 34 (CCDC34) and its Prognostic Significance in Pancreatic Adenocarcinoma. Med Sci Monit. 2017;23:6012–8.
Geng W, Liang W, Fan Y, Ye Z, Zhang L. Overexpression of CCDC34 in colorectal cancer and its involvement in tumor growth, apoptosis and invasion. Mol Med Rep. 2018;17:465–73.
Liu LB, Huang J, Zhong JP, Ye GL, Xue L, Zhou MH, et al. High Expression of CCDC34 Is Associated with Poor Survival in Cervical Cancer Patients. Med Sci Monit. 2018;24:8383–90.
Gong Y, Qiu W, Ning X, Yang X, Liu L, Wang Z, et al. CCDC34 is up-regulated in bladder cancer and regulates bladder cancer cell proliferation, apoptosis and migration. Oncotarget. 2015;6:25856–67.
Hu DD, Li PC, He YF, Jia W, Hu B. Overexpression of Coiled-Coil Domain-Containing Protein 34 (CCDC34) and its Correlation with Angiogenesis in Esophageal Squamous Cell Carcinoma. Med Sci Monit. 2018;24:698–705.
Lin Z, Qu S, Peng W, Yang P, Zhang R, Zhang P, et al. Up-Regulated CCDC34 Contributes to the Proliferation and Metastasis of Hepatocellular Carcinoma. Onco Targets Ther. 2020;13:51–60.
Zhou M, Chen X, Bai H, Sun Y, Zhang Z, Li S, et al. RABL2A-CCDC34 Axis Promotes Sorafenib Resistance in Hepatocellular Carcinoma. DNA Cell Biol. 2021;40:1418–27.
Wu J, Yue C, Xu W, Li H, Zhu J, Li L. MNX1 facilitates the malignant progress of lung adenocarcinoma through transcriptionally upregulating CCDC34. Oncol Lett. 2023;26:325.
Malta TM, Sokolov A, Gentles AJ, Burzykowski T, Poisson L, Weinstein JN, et al. Machine Learning Identifies Stemness Features Associated with Oncogenic Dedifferentiation. Cell. 2018;173:338–.e15.
Liu D, Lin L, Wang Y, Chen L, He Y, Luo Y, et al. PNO1, which is negatively regulated by miR-340-5p, promotes lung adenocarcinoma progression through Notch signaling pathway. Oncogenesis. 2020;9:58.
Dev A Jr, Vachher M, Prasad CP. β-catenin inhibitors in cancer therapeutics: intricacies and way forward. Bioengineered. 2023. 14: 2251696.
Gajos-Michniewicz A, Czyz M. WNT/β-catenin signaling in hepatocellular carcinoma: The aberrant activation, pathogenic roles, and therapeutic opportunities. Genes Dis. 2024;11:727–46.
Wang J, Yu H, Dong W, Zhang C, Hu M, Ma W, et al. N6-Methyladenosine-Mediated Up-Regulation of FZD10 Regulates Liver Cancer Stem Cells’ Properties and Lenvatinib Resistance Through WNT/β-Catenin and Hippo Signaling Pathways. Gastroenterology. 2023;164:990–1005.
Klionsky DJ, Petroni G, Amaravadi RK, Baehrecke EH, Ballabio A, Boya P, et al. Autophagy in major human diseases. EMBO J. 2021;40:e108863.
Debnath J, Gammoh N, Ryan KM. Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol. 2023;24:560–75.
Wang X, Lee J, Xie C. Autophagy Regulation on Cancer Stem Cell Maintenance, Metastasis, and Therapy Resistance. Cancers (Basel). 2022;14:381.
Mo L, Su B, Xu L, Hu Z, Li H, Du H, et al. MCM7 supports the stemness of bladder cancer stem-like cells by enhancing autophagic flux. iScience. 2022;25:105029.
Liu Y, Li S, Wang S, Yang Q, Wu Z, Zhang M, et al. LIMP-2 enhances cancer stem-like cell properties by promoting autophagy-induced GSK3β degradation in head and neck squamous cell carcinoma. Int J Oral Sci. 2023;15:24.
Zhou C, Liang Y, Zhou L, Yan Y, Liu N, Zhang R, et al. TSPAN1 promotes autophagy flux and mediates cooperation between WNT-CTNNB1 signaling and autophagy via the MIR454-FAM83A-TSPAN1 axis in pancreatic cancer. Autophagy. 2021;17:3175–95.
Zhao YG, Codogno P, Zhang H. Machinery, regulation and pathophysiological implications of autophagosome maturation. Nat Rev Mol Cell Biol. 2021;22:733–50.
Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med. 2021;27:1345–56.
Yan D, Huelse JM, Kireev D, Tan Z, Chen L, Goyal S, et al. MERTK activation drives osimertinib resistance in EGFR-mutant non-small cell lung cancer. J Clin Invest. 2022;132:e150517.
Kim N, Kim HK, Lee K, Hong Y, Cho JH, Choi JW, et al. Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma. Nat Commun. 2020;11:2285.
Zalaquett Z, Catherine Rita Hachem M, Kassis Y, Hachem S, Eid R, Raphael Kourie H, et al. Acquired resistance mechanisms to osimertinib: The constant battle. Cancer Treat Rev. 2023;116:102557.
Murciano-Goroff YR, Taylor BS, Hyman DM, Schram AM. Toward a More Precise Future for Oncology. Cancer Cell. 2020;37:431–42.
Liu Q, Yu S, Zhao W, Qin S, Chu Q, Wu K. EGFR-TKIs resistance via EGFR-independent signaling pathways. Mol Cancer. 2018;17:53.
Si J, Ma Y, Bi JW, Xiong Y, Lv C, Li S, et al. Shisa3 brakes resistance to EGFR-TKIs in lung adenocarcinoma by suppressing cancer stem cell properties. J Exp Clin Cancer Res. 2019;38:481.
Jiang P, Li Y, Poleshko A, Medvedeva V, Baulina N, Zhang Y, et al. The Protein Encoded by the CCDC170 Breast Cancer Gene Functions to Organize the Golgi-Microtubule Network. EBioMedicine. 2017;22:28–43.
Zeng Z, Fu M, Hu Y, Wei Y, Wei X, Luo M. Regulation and signaling pathways in cancer stem cells: implications for targeted therapy for cancer. Mol Cancer. 2023;22:172.
Nakayama S, Sng N, Carretero J, Welner R, Hayashi Y, Yamamoto M, et al. β-catenin contributes to lung tumor development induced by EGFR mutations. Cancer Res. 2014;74:5891–902.
Li K, Peng ZY, Wang R, Li X, Du N, Liu DP, et al. Enhancement of TKI sensitivity in lung adenocarcinoma through m6A-dependent translational repression of Wnt signaling by circ-FBXW7. Mol Cancer. 2023;22:103.
Ma Q, Yu J, Zhang X, Wu X, Deng G. Wnt/β-catenin signaling pathway-a versatile player in apoptosis and autophagy. Biochimie. 2023;211:57–67.
Xiong XX, Hu DX, Xu L, Lin H, Zhang Y, Li CY, et al. Selective 14-3-3γ Upregulation Promotes Beclin-1-LC3-Autophagic Influx via β-Catenin Interaction in Starved Neurons In Vitro and In Vivo. Neurochem Res. 2019;44:849–58.
Zhu Y, Huang S, Chen S, Chen J, Wang Z, Wang Y, et al. SOX2 promotes chemoresistance, cancer stem cells properties, and epithelial-mesenchymal transition by β-catenin and Beclin1/autophagy signaling in colorectal cancer. Cell Death Dis. 2021;12:449.
Zhang R, Li SW, Liu L, Yang J, Huang G, Sang Y. TRIM11 facilitates chemoresistance in nasopharyngeal carcinoma by activating the β-catenin/ABCC9 axis via p62-selective autophagic degradation of Daple. Oncogenesis. 2020;9:45.
Wu X, Zhang J, Ma C, Li W, Zeng J, Wang Y, et al. A role for Wnt/β-catenin signalling in suppressing Bacillus Calmette-Guerin-induced macrophage autophagy. Micro Pathog. 2019;127:277–87.
Shen W, Luo P, Sun Y, Zhang W, Zhou N, Zhan H, et al. NRBF2 regulates the chemoresistance of small cell lung cancer by interacting with the P62 protein in the autophagy process. iScience. 2022;25:104471.
Yang Y, Klionsky DJ. Autophagy and disease: unanswered questions. Cell Death Differ. 2020;27:858–71.
Li L, Wang Y, Jiao L, Lin C, Lu C, Zhang K, et al. Protective autophagy decreases osimertinib cytotoxicity through regulation of stem cell-like properties in lung cancer. Cancer Lett. 2019;452:191–202.
Cao P, Li Y, Shi R, Yuan Y, Gong H, Zhu G, et al. Combining EGFR-TKI With SAHA Overcomes EGFR-TKI-Acquired Resistance by Reducing the Protective Autophagy in Non-Small Cell Lung Cancer. Front Chem. 2022;10:837987.
Ning Y, Zheng H, Yang Y, Zang H, Wang W, Zhan Y, et al. YAP1 synergize with YY1 transcriptional co-repress DUSP1 to induce osimertinib resistant by activating the EGFR/MAPK pathway and abrogating autophagy in non-small cell lung cancer. Int J Biol Sci. 2023;19:2458–74.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 82173208), the Key Project of Science & Technology Development Fund of Tianjin Education Commission for Higher Education (No. 2022ZD064) and Tianjin Key Medical Discipline (Specialty) Constrction Project (TJYXZDXK-010A).
Author information
Authors and Affiliations
Contributions
HG, YH and PY conceived the study and designed the experiments. PY, YH, RZ, WG, YW, YL, LC, YF, and YG performed the experiments and analyzed the data. HG, PC, LZ supervised the study and provided insightful discussion on this study. PY wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was conducted in accordance with Declaration of Helsinki, and all experiments were approved by the Ethics Committee of Tianjin Medical University Cancer Hospital (approval number: EK20240014). All participants have provided written informed consent to take part in the study.
Consent for publication
All authors have approved the manuscript to submission.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yue, P., He, Y., Zuo, R. et al. CCDC34 maintains stemness phenotype through β-catenin-mediated autophagy and promotes EGFR-TKI resistance in lung adenocarcinoma. Cancer Gene Ther 32, 104–121 (2025). https://doi.org/10.1038/s41417-024-00843-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41417-024-00843-y
This article is cited by
-
5-HT regulates resistance to aumolertinib by attenuating ferroptosis in lung adenocarcinoma
EMBO Molecular Medicine (2025)
-
The oncogenic role of CCDC34 in lower-grade gliomas: prognostic significance and therapeutic potential
Journal of Neuro-Oncology (2025)
-
Silencing of telomerase RNA component induces autophagy and ferroptosis in A549 and H838 lung cancer cells via AMPK-mediated signaling
Molecular and Cellular Biochemistry (2025)