Abstract
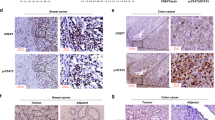
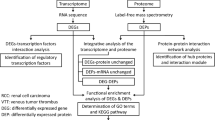
Despite significant advancements in diagnosis and treatment, cancer remains a leading cause of mortality globally. Cancer gene therapy has emerged as a promising strategy, with numerous clinical trials demonstrating its efficacy in targeting tumor cells, vasculature, and immune components. However, precise and selective gene expression regulation remains a challenge. In this study, we demonstrated that the CREPT (cell-cycle related and expression-elevated protein in tumor) promoter holds great potential for targeted cancer gene therapy. CREPT, a tumor-promoting protein as a positive regulator of gene transcription, is highly expressed across various cancer types while exhibiting minimal expression in normal tissues. The CREPT promoter mediated robust and tumor-selective transgene expression in vivo following both local and systemic administration, with only minimal off-target expression detected in blood and none in normal organs. Leveraging this specificity, we engineered an adenovirus encoding diphtheria toxin fragment A under CREPT promoter regulation. This construct was selectively expressed and inhibited protein synthesis, leading cancer cell death in vitro and in vivo. These findings demonstrate that the CREPT promoter may serve as a useful regulatory element for the development of targeted cancer gene therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Ferlay J EM, Lam F, Colombet M, Mery L, Pineros M, et al. Global cancer observatory: cancer today 2021 [Available from: https://gco.iarc.fr/today.
Hardee CL, Arévalo-Soliz LM, Hornstein BD, Zechiedrich L. Advances in non-viral DNA vectors for gene therapy. Genes. 2017;8:541–55. https://doi.org/10.3390/genes8020065.
Alderuccio F, Chan J, Toh B-H. Tweaking the immune system: gene therapy-assisted autologous haematopoietic stem cell transplantation as a treatment for autoimmune disease. Autoimmunity. 2008;41:679–85. https://doi.org/10.1080/08916930802197123.
Okegawa T, Pong R-C, Li Y, Hsieh J-T. The role of cell adhesion molecule in cancer progression and its application in cancer therapy. Acta Biochim Pol. 2004;51:445–57.
Persano L, Crescenzi M, Indraccolo S. Anti-angiogenic gene therapy of cancer: current status and future prospects. Mol Asp Med. 2007;28:87–114.
Esteva FJ, Hubbard-Lucey VM, Tang J, Pusztai L. Immunotherapy and targeted therapy combinations in metastatic breast cancer. Lancet Oncol. 2019;20:e175–e86. https://doi.org/10.1016/S1470-2045(19)30026-9.
Lazzari C, Bulotta A, Damiano G, Mirabile A, Viganó M, Veronesi G, et al. Angiogenesis inhibition in lung cancer: emerging novel strategies. Curr Opin Oncol. 2022;34:107–14. https://doi.org/10.1097/CCO.0000000000000807.
Xia Y, Du Z, Wang X, Li X. Treatment of uterine sarcoma with rAd-p53 (Gendicine) followed by chemotherapy: clinical study of TP53 gene therapy. Hum Gene Ther. 2018;29:242–50. https://doi.org/10.1089/hum.2017.206.
Hardcastle J, Kurozumi K, Chiocca EA, Kaur B. Oncolytic viruses driven by tumor-specific promoters. Curr Cancer Drug Targets. 2007;7:181–9.
Shafiee F, Aucoin MG, Jahanian-Najafabadi A. Targeted diphtheria toxin-based therapy: a review article. Front Microbiol. 2019;10:2340. https://doi.org/10.3389/fmicb.2019.02340.
Holmes RK. Biology and molecular epidemiology of diphtheria toxin and the tox gene. J Infect Dis. 2000;181:S156–S67.
Li M, Ma D, Chang Z. Current understanding of CREPT and p15RS, carboxy-terminal domain (CTD)-interacting proteins, in human cancers. Oncogene. 2021;40:705–16. https://doi.org/10.1038/s41388-020-01544-0.
Li M, Li J, He C, Jiang G, Ma D, Guan H, et al. An oncoprotein CREPT functions as a co-factor in MYC-driven transformation and tumor growth. J Biol Chem. 2025;301:108030. https://doi.org/10.1016/j.jbc.2024.108030.
Li J, Xu L, Wang J, Wang X, Lin Y, Zou AY, et al. CREPT is required for the metastasis of triple-negative breast cancer through a co-operational-chromatin loop-based gene regulation. Mol Cancer. 2025;24:170. https://doi.org/10.1186/s12943-025-02361-3.
Lu D, Wu Y, Wang Y, Ren F, Wang D, Su F, et al. CREPT accelerates tumorigenesis by regulating the transcription of cell-cycle-related genes. Cancer Cell. 2012;21:92–104. https://doi.org/10.1016/j.ccr.2011.12.016.
Wang Y, Qiu H, Hu W, Li S, Yu J. RPRD1B promotes tumor growth by accelerating the cell cycle in endometrial cancer. Oncol Rep. 2014;31:1389–95. https://doi.org/10.3892/or.2014.2990.
Liu T, Li W-M, Wang W-P, Sun Y, Ni Y-F, Xing H, et al. Inhibiting CREPT reduces the proliferation and migration of non-small cell lung cancer cells by down-regulating cell cycle related protein. Am J Transl Res. 2016;8:2097–113.
Zhang Y, Wang S, Kang W, Liu C, Dong Y, Ren F, et al. CREPT facilitates colorectal cancer growth through inducing Wnt/β-catenin pathway by enhancing p300-mediated β-catenin acetylation. Oncogene. 2018;37:3485–500. https://doi.org/10.1038/s41388-018-0161-z.
Cao Y, Ning B, Tian Y, Lan T, Chu Y, Ren F, et al. CREPT disarms the inhibitory Activity of HDAC1 on oncogene expression to promote tumorigenesis. Cancers. 2022;14:4797. https://doi.org/10.3390/cancers14194797.
Motea EA, Fattah FJ, Xiao L, Girard L, Rommel A, Morales JC, et al. Kub5-Hera RPRD1B deficiency promotes “BRCAness” and vulnerability to PARP inhibition in BRCA-proficient breast cancers. Clin Cancer Res. 2018;24:6459–70. https://doi.org/10.1158/1078-0432.CCR-17-1118.
Ding L, Yang L, He Y, Zhu B, Ren F, Fan X, et al. CREPT/RPRD1B associates with Aurora B to regulate Cyclin B1 expression for accelerating the G2/M transition in gastric cancer. Cell Death Dis. 2018;9:1172. https://doi.org/10.1038/s41419-018-1211-8.
Ma J, Ren Y, Zhang L, Kong X, Wang T, Shi Y, et al. Knocking-down of CREPT prohibits the progression of oral squamous cell carcinoma and suppresses cyclin D1 and c-Myc expression. PLoS ONE. 2017;12:e0174309. https://doi.org/10.1371/journal.pone.0174309.
Wei M, Cao Y, Jia D, Zhao H, Zhang L. CREPT promotes glioma cell proliferation and invasion by activating Wnt/β-catenin pathway and is a novel target of microRNA-596. Biochimie. 2019;162:116–24. https://doi.org/10.1016/j.biochi.2019.04.014.
Yu S, Huang H, Wang S, Xu H, Xue Y, Huang Y, et al. CREPT is a novel predictor of the response to adjuvant therapy or concurrent chemoradiotherapy in esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2019;12:3301–10.
Wen N, Bian L, Gong J, Meng Y. Overexpression of cell-cycle related and expression-elevated protein in tumor (CREPT) in malignant cervical cancer. J Int Med Res. 2020;48:300060519895089. https://doi.org/10.1177/0300060519895089.
Li M, Lin Y, Wang J, Yang H, Ma D, Tian Y, et al. CREPT promotes LUAD progression by enhancing the CDK9 and RNAPII assembly to promote ERK-driven gene transcription. Theranostics. 2025;15:8337–59. https://doi.org/10.7150/thno.115572.
Farooq U, Li J, Chang Z. Disrupting cell cycle machinery: CREPT is an emerging target in cancer therapy. Cancers. 2025;17:2401 https://doi.org/10.3390/cancers17142401.
Wu Y, Zhang Y, Zhang H, Yang X, Wang Y, Ren F, et al. p15RS attenuates Wnt/{beta}-catenin signaling by disrupting {beta}-catenin·TCF4 Interaction. J Biol Chem. 2010;285:34621–31. https://doi.org/10.1074/jbc.M110.148791.
Lin Y, Jiang H, Li J, Ren F, Wang Y, Qiu Y, et al. Microenvironment-induced CREPT expression by cancer-derived small extracellular vesicles primes field cancerization. Theranostics. 2024;14:662–80. https://doi.org/10.7150/thno.87344.
Zhang Y, Liu C, Duan X, Ren F, Li S, Jin Z, et al. CREPT/RPRD1B, a recently identified novel protein highly expressed in tumors, enhances the β-catenin·TCF4 transcriptional activity in response to Wnt signaling. J Biol Chem. 2014;289:22589–99. https://doi.org/10.1074/jbc.M114.560979.
Rong Y, Cheng L, Ning H, Zou J, Zhang Y, Xu F, et al. Wilms’ tumor 1 and signal transducers and activators of transcription 3 synergistically promote cell proliferation: a possible mechanism in sporadic Wilms’ tumor. Cancer Res. 2006;66:8049–57.
Hine CM, Seluanov A, Gorbunova V. Use of the Rad51 promoter for targeted anti-cancer therapy. Proc Natl Acad Sci USA. 2008;105:20810–5. https://doi.org/10.1073/pnas.0807990106.
Mattheakis LC, Sor F, Collier RJ. Diphthamide synthesis in Saccharomyces cerevisiae: structure of the DPH2 gene. Gene. 1993;132:149–54.
Mattheakis LC, Shen WH, Collier RJ. DPH5, a methyltransferase gene required for diphthamide biosynthesis in Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:4026–37.
Liu S, Milne GT, Kuremsky JG, Fink GR, Leppla SH. Identification of the proteins required for biosynthesis of diphthamide, the target of bacterial ADP-ribosylating toxins on translation elongation factor 2. Mol Cell Biol. 2004;24:9487–97.
Ohana P, Bibi O, Matouk I, Levy C, Birman T, Ariel I, et al. Use of H19 regulatory sequences for targeted gene therapy in cancer. Int J Cancer. 2002;98:645–50.
Ohana P, Schachter P, Ayesh B, Mizrahi A, Birman T, Schneider T, et al. Regulatory sequences of H19 and IGF2 genes in DNA-based therapy of colorectal rat liver metastases. J Gene Med. 2005;7:366–74.
Hine CM, Seluanov A, Gorbunova V. Rad51 promoter-targeted gene therapy is effective for in vivo visualization and treatment of cancer. Mol Ther. 2012;20:347–55. https://doi.org/10.1038/mt.2011.215.
Chen Y, Li Z, Xu Z, Tang H, Guo W, Sun X, et al. Use of the XRCC2 promoter for in vivo cancer diagnosis and therapy. Cell Death Dis. 2018;9:420. https://doi.org/10.1038/s41419-018-0453-9.
Peng W, Chen J, Huang YH, Sawicki JA. Tightly-regulated suicide gene expression kills PSA-expressing prostate tumor cells. Gene Ther. 2005;12:1573–80.
Abdul-Ghani R, Ohana P, Matouk I, Ayesh S, Ayesh B, Laster M, et al. Use of transcriptional regulatory sequences of telomerase (hTER and hTERT) for selective killing of cancer cells. Mol Ther. 2000;2:539–44.
Sidi AA, Ohana P, Benjamin S, Shalev M, Ransom JH, Lamm D, et al. Phase I/II marker lesion study of intravesical BC-819 DNA plasmid in H19 over expressing superficial bladder cancer refractory to bacillus Calmette-Guerin. J Urol. 2008;180:2379–83. https://doi.org/10.1016/j.juro.2008.08.006.
Gofrit ON, Benjamin S, Halachmi S, Leibovitch I, Dotan Z, Lamm DL, et al. DNA based therapy with diphtheria toxin-A BC-819: a phase 2b marker lesion trial in patients with intermediate risk nonmuscle invasive bladder cancer. J Urol. 2014;191:1697–702. https://doi.org/10.1016/j.juro.2013.12.011.
Ma D, Zou Y, Chu Y, Liu Z, Liu G, Chu J, et al. A cell-permeable peptide-based PROTAC against the oncoprotein CREPT proficiently inhibits pancreatic cancer. Theranostics. 2020;10:3708–21. https://doi.org/10.7150/thno.41677.
Lan T, Li M, Duan X, Jia H, Cao Y, Wang Y, et al. Regulation of revival stem cell differentiation by CREPT/RPRD1B during intestinal regeneration. Cell Biosci. 2025;15:98. https://doi.org/10.1186/s13578-025-01434-6.
Yang L, Yang H, Chu Y, Song Y, Ding L, Zhu B, et al. CREPT is required for murine stem cell maintenance during intestinal regeneration. Nat Commun. 2021;12:270. https://doi.org/10.1038/s41467-020-20636-9.
Wang M, Wu B, Tang K, Wang X, Liu X, Duan Y, et al. Cell-cycle-related and expression elevated protein in tumor upregulates the antioxidant genes via activation of NF-κB/Nrf2 in acute liver injury. Toxics. 2024;12:893. https://doi.org/10.3390/toxics12120893.
Heise C, Hermiston T, Johnson L, Brooks G, Sampson-Johannes A, Williams A, et al. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med. 2000;6:1134–9.
Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12.
Lang FF, Conrad C, Gomez-Manzano C, Yung WKA, Sawaya R, Weinberg JS, et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol. 2018;36:1419–27. https://doi.org/10.1200/JCO.2017.75.8219.
Ulasov IV, Tyler MA, Rivera AA, Nettlebeck DM, Douglas JT, Lesniak MS. Evaluation of E1A double mutant oncolytic adenovectors in anti-glioma gene therapy. J Med Virol. 2008;80:1595–603. https://doi.org/10.1002/jmv.21264.
Lau L, Gray EE, Brunette RL, Stetson DB. DNA tumor virus oncogenes antagonize the cGAS-STING DNA-sensing pathway. Science. 2015;350:568–71. https://doi.org/10.1126/science.aab3291.
Kent LN, Leone G. The broken cycle: E2F dysfunction in cancer. Nat Rev Cancer. 2019;19:326–38. https://doi.org/10.1038/s41568-019-0143-7.
Kitajima S, Li F, Takahashi C. Tumor milieu controlled by rb tumor suppressor. Int J Mol Sci. 2020;21:2450. https://doi.org/10.3390/ijms21072450.
Koch P, Gatfield J, Löber C, Hobom U, Lenz-Stöppler C, Roth J, et al. Efficient replication of adenovirus despite the overexpression of active and nondegradable p53. Cancer Res. 2001;61:5941–7.
O’Shea CC, Johnson L, Bagus B, Choi S, Nicholas C, Shen A, et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–23.
Ramachandra M, Rahman A, Zou A, Vaillancourt M, Howe JA, Antelman D, et al. Re-engineering adenovirus regulatory pathways to enhance oncolytic specificity and efficacy. Nat Biotechnol. 2001;19:1035–41.
Gürlevik E, Woller N, Schache P, Malek NP, Wirth TC, Zender L, et al. p53-dependent antiviral RNA-interference facilitates tumor-selective viral replication. Nucleic Acids Res. 2009;37:e84. https://doi.org/10.1093/nar/gkp374.
Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–6.
Koodie L, Robertson MG, Chandrashekar M, Ruth G, Dunning M, Bianco RW, et al. Rodents versus pig model for assessing the performance of serotype chimeric Ad5/3 oncolytic adenoviruses. Cancers. 2019;11:198. https://doi.org/10.3390/cancers11020198.
Zafar S, Quixabeira DCA, Kudling TV, Cervera-Carrascon V, Santos JM, Grönberg-Vähä-Koskela S, et al. Ad5/3 is able to avoid neutralization by binding to erythrocytes and lymphocytes. Cancer Gene Ther. 2021;28:442–54. https://doi.org/10.1038/s41417-020-00226-z.
Amit D, Tamir S, Birman T, Gofrit ON, Hochberg A. Development of targeted therapy for bladder cancer mediated by a double promoter plasmid expressing diphtheria toxin under the control of IGF2-P3 and IGF2-P4 regulatory sequences. Int J Clin Exp Med. 2011;4:91–102.
Acknowledgements
We thank Kuancan Liu from Central Laboratory, Xiang’an Hospital, School of Medicine, Xiamen University, Xiamen, Fujian, China for providing plasmid pBSDT-AII.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82430087 to ZC) and grants from the Science and Technology Bureau of Jiaxing city, Zhejiang, China (2024AZ30004 to SJ).
Author information
Authors and Affiliations
Contributions
JL, LMD, ZC, and FR conceived and designed the project. JL, WW, and GJ designed and performed experiments. JL acquired, analyzed, and visualized data. JL wrote the original draft of the paper. LMD, YW, SJQ, ZC, SJ, LMY and FR revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All animal experiments were performed in accordance with relevant guidelines and regulations and were approved by the Institutional Animal Care and Use Committee of Tsinghua University. The animal work was conducted under the protocol 20-CZJ-2. The research involving human participants was approved by the Clinical Ethics Committee of the Chinese PLA General Hospital (Approval Number: S2022-610-01). Informed consent was obtained from all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Li, M., Wang, W. et al. Targeted suppression of tumor growth by CREPT promoter-driven diphtheria toxin fragment A. Cancer Gene Ther 33, 145–154 (2026). https://doi.org/10.1038/s41417-025-00961-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41417-025-00961-1