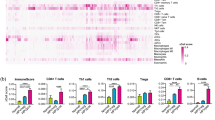
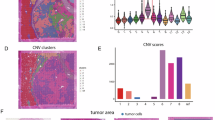
Abstract
Tertiary lymphoid structures (TLS) are associated with an improved response to Immune checkpoint therapy (ICT) in head and neck squamous cell carcinoma (HNSCC). Human papillomavirus (HPV) infection constitutes a high-risk factor for HNSCC carcinogenesis. However, its role in TLS formation has yet to be elucidated. Herein, immunohistochemical (IHC) analysis from 59 HNSCC patients revealed a higher prevalence of mature TLS in HPV-positive (HPV+) HNSCC compared to HPV-negative (HPV-) cases. Furthermore, integrated analysis of single-cell RNA sequencing, spatial transcriptomics, and RNA-seq data indicated that TLS-positive tumors were characterized by an expanded population of KRT15high tumor cells in HNSCC. IHC and cytological experiments confirmed upregulation of KRT15 in HPV+HNSCC tumor cells, which also showed high expression of cancer stem cell marker genes. These KRT15high stem-like tumor cells specifically secreted CCL20, which was related to the infiltration of TLS-associated immune cells in HPV+HNSCC. Murine models confirmed that CCL20 treatment promoted TLS formation and enhanced the efficacy of anti-PD-1 therapy. Multiplex immunofluorescence showed that TLS provided specialized microenvironments that supported the proliferation of CD39+PD-1+CD8+T cells. Collectively, our findings proposed that CCL20 secreted by HPV-infected KRT15high tumor cells promoted TLS formation, thereby enhancing anti-PD-1 therapy responses in HPV+HNSCC. This study provides mechanistic insights into HPV-mediated TLS development and supports precision immunotherapeutic strategies for HNSCC.
Similar content being viewed by others
Data availability
The scRNA-seq data analyzed in this study were obtained from the Gene Expression Omnibus (GEO) at GSE139324 and GSE164690. RNA-seq data were obtained from The Cancer Genome Atlas (TCGA). The ST-seq data generated in this study are available upon request from the corresponding author.
References
Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6:92.
Tumban E. A current update on human papillomavirus-associated head and neck cancers. Viruses. 2019;11:922
Bhatia A, Burtness B. Treating head and neck cancer in the age of immunotherapy: a 2023 update. Drugs. 2023;83:217–48.
Ruffin AT, Cillo AR, Tabib T, Liu A, Onkar S, Kunning SR, et al. B cell signatures and tertiary lymphoid structures contribute to outcome in head and neck squamous cell carcinoma. Nat Commun. 2021;12:3349.
Iglesia MD, Parker JS, Hoadley KA, Serody JS, Perou CM, Vincent BG. Genomic analysis of immune cell infiltrates across 11 tumor types. J Natl Cancer Inst. 2016; 108:djw144.
Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–95.
Kim SS, Shen S, Miyauchi S, Sanders PD, Franiak-Pietryga I, Mell L, et al. B cells improve overall survival in HPV-associated squamous cell carcinomas and are activated by radiation and PD-1 blockade. Clin Cancer Res. 2020;26:3345–59.
Sautes-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19:307–25.
Zhang Y, Xu M, Ren Y, Ba Y, Liu S, Zuo A, et al. Tertiary lymphoid structural heterogeneity determines tumour immunity and prospects for clinical application. Mol Cancer. 2024;23:75.
Posch F, Silina K, Leibl S, Mundlein A, Moch H, Siebenhuner A, et al. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. Oncoimmunology. 2018;7:e1378844.
Wishnie AJ, Chwat-Edelstein T, Attaway M, Vuong BQ. BCR affinity influences T-B interactions and B cell development in secondary lymphoid organs. Front Immunol. 2021;12:703918.
Matlung SE, Wilhelmina van Kempen PM, Bovenschen N, van Baarle D, Willems SM. Differences in T-cell infiltrates and survival between HPV+ and HPV- oropharyngeal squamous cell carcinoma. Future Sci OA. 2016;2:FSO88.
Schumacher TN, Thommen DS. Tertiary lymphoid structures in cancer. Science. 2022;375:eabf9419.
Zhao L, Jin S, Wang S, Zhang Z, Wang X, Chen Z, et al. Tertiary lymphoid structures in diseases: immune mechanisms and therapeutic advances. Signal Transduct Target Ther. 2024;9:225.
Li JP, Wu CY, Chen MY, Liu SX, Yan SM, Kang YF, et al. PD-1(+)CXCR5(-)CD4(+) Th-CXCL13 cell subset drives B cells into tertiary lymphoid structures of nasopharyngeal carcinoma. J Immunother Cancer. 2021;9:e002101.
Zhang Y, Liu G, Zeng Q, Wu W, Lei K, Zhang C, et al. CCL19-producing fibroblasts promote tertiary lymphoid structure formation enhancing anti-tumor IgG response in colorectal cancer liver metastasis. Cancer Cell. 2024;42:1370–85.e9.
Zhang S, Wang B, Ma F, Tong F, Yan B, Liu T, et al. Characteristics of B lymphocyte infiltration in HPV(+) head and neck squamous cell carcinoma. Cancer Sci. 2021;112:1402–16.
Jin S, Guerrero-Juarez CF, Zhang L, Chang I, Ramos R, Kuan CH, et al. Inference and analysis of cell-cell communication using CellChat. Nat Commun. 2021;12:1088.
Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48:W509–W14.
Hamilton M, Mars Z, Sedeuil M, Rolland M, Jean D, Boudreau F, et al. ASCL2 is a key regulator of the proliferation-differentiation equilibrium in the esophageal epithelium. Biol Open. 2024;13:bio059919.
Yuan X, Yi M, Dong B, Chu Q, Wu K. Prognostic significance of KRT19 in lung squamous cancer. J Cancer. 2021;12:1240–8.
Losquadro WD. Anatomy of the skin and the pathogenesis of nonmelanoma skin cancer. Facial Plast Surg Clin North Am. 2017;25:283–9.
Kadomoto S, Izumi K, Mizokami A. The CCL20-CCR6 axis in cancer progression. Int J Mol Sci. 2020;21:5186.
Patil NS, Nabet BY, Muller S, Koeppen H, Zou W, Giltnane J, et al. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung cancer. Cancer Cell. 2022;40:289–300.e4.
Hugaboom MB, Wirth LV, Street K, Ruthen N, Jegede OA, Schindler NR, et al. Presence of tertiary lymphoid structures and exhausted tissue-resident T cells determines clinical response to PD-1 blockade in renal cell carcinoma. Cancer Discov. 2025;15:948–68.
Laba S, Mallett G, Amarnath S. The depths of PD-1 function within the tumor microenvironment beyond CD8(+) T cells. Semin Cancer Biol. 2022;86:1045–55.
Chow A, Uddin FZ, Liu M, Dobrin A, Nabet BY, Mangarin L, et al. The ectonucleotidase CD39 identifies tumor-reactive CD8(+) T cells predictive of immune checkpoint blockade efficacy in human lung cancer. Immunity. 2023;56:93–106 e6.
Koh CH, Lee S, Kwak M, Kim BS, Chung Y. CD8 T-cell subsets: heterogeneity, functions, and therapeutic potential. Exp Mol Med. 2023;55:2287–99.
Bayik D, Lathia JD. Cancer stem cell-immune cell crosstalk in tumour progression. Nat Rev Cancer. 2021;21:526–36.
Giroux V, Lento AA, Islam M, Pitarresi JR, Kharbanda A, Hamilton KE, et al. Long-lived keratin 15+ esophageal progenitor cells contribute to homeostasis and regeneration. J Clin Invest. 2017;127:2378–91.
Ievlev V, Lynch TJ, Freischlag KW, Gries CB, Shah A, Pai AC, et al. Krt14 and Krt15 differentially regulate regenerative properties and differentiation potential of airway basal cells. JCI Insight. 2023;8:e162041.
Martins LR, Sieverling L, Michelhans M, Schiller C, Erkut C, Grunewald TGP, et al. Single-cell division tracing and transcriptomics reveal cell types and differentiation paths in the regenerating lung. Nat Commun. 2024;15:2246.
Tommasi C, Yogev O, Yee MB, Drousioti A, Jones M, Ring A, et al. Upregulation of keratin 15 is required for varicella-zoster virus replication in keratinocytes and is attenuated in the live attenuated vOka vaccine strain. Virol J. 2024;21:253.
da Silva-Diz V, Sole-Sanchez S, Valdes-Gutierrez A, Urpi M, Riba-Artes D, Penin RM, et al. Progeny of Lgr5-expressing hair follicle stem cell contributes to papillomavirus-induced tumor development in epidermis. Oncogene. 2013;32:3732–43.
Jain M, Yadav D, Jarouliya U, Chavda V, Yadav AK, Chaurasia B, et al. Epidemiology, molecular pathogenesis, immuno-pathogenesis, immune escape mechanisms and vaccine evaluation for HPV-associated carcinogenesis. Pathogens. 2023;12:1380.
Zhong P, Shu R, Wu H, Liu Z, Shen X, Hu Y. Low KRT15 expression is associated with poor prognosis in patients with breast invasive carcinoma. Exp Ther Med. 2021;21:305.
Yang H, Li A, Li A, Zhao F, Zhang T. Upregulated keratin 15 links to the occurrence of lymphovascular invasion, stromal cervical invasion as well as unfavorable survival profile in endometrial cancer patients. Medicine. 2022;101:e29686.
Zhou TL, Chen HX, Wang YZ, Wen SJ, Dao PH, Wang YH, et al. Single-cell RNA sequencing reveals the immune microenvironment and signaling networks in cystitis glandularis. Front Immunol. 2023;14:1083598.
Ward-Kavanagh LK, Lin WW, Sedy JR, Ware CF. The TNF receptor superfamily in co-stimulating and co-inhibitory responses. Immunity. 2016;44:1005–19.
Beatty GL, Gladney WL. Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res. 2015;21:687–92.
Lee AYS, Korner H. The CCR6-CCL20 axis in humoral immunity and T-B cell immunobiology. Immunobiology. 2019;224:449–54.
Walch-Ruckheim B, Mavrova R, Henning M, Vicinus B, Kim YJ, Bohle RM, et al. Stromal fibroblasts induce CCL20 through IL6/C/EBPbeta to support the recruitment of Th17 cells during cervical cancer progression. Cancer Res. 2015;75:5248–59.
Acknowledgements
We would like to thank the Center for Computational Science and Engineering at Southern University of Science and Technology for technical support.
Funding
This study was supported by the following funding sources: the Shenzhen Science and Technology Program (JCYJ20240813101805007 and JCYJ20240813101802004); the National Natural Science Foundation of China (No. 82473326); and the Guangdong Province Basic and Applied Basic Research Fund Project (2023A1515220104).
Author information
Authors and Affiliations
Contributions
SZ and YJ were responsible for the writing and revision of the manuscript. SZ also contributed to the overall bioinformatics analysis. HL performed animal model generation and associated experimental procedures. XL was responsible for pathological area classification and paraffin tissue embedding. LT and TL contributed to immunohistochemistry experiments. RL and ZL were responsible for scRNA-seq data analysis. MW, JS, and ZH contributed to the collection of HNSCC tissues and clinical information. HM and LW were responsible for the overall study design, coordination of the research, and establishment of the research objectives. LW also contributed to the revision of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
The experimental protocol was established according to the ethical guidelines of the Helsinki Declaration and was approved by the Human Ethics Committee of the Third People’s Hospital in Shenzhen [No. 2025-016]. All animals’ experimental procedures received ethical approval for the animal research from the Ethics Committee in the Third People’s Hospital in Shenzhen [2025-017-01].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Yufang Shi
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, S., Liu, H., Li, X. et al. CCL20 secreted by KRT15high tumor Cells promotes tertiary lymphoid structure formation and enhances anti-PD-1 therapy response in HPV+HNSCC. Cell Death Dis (2025). https://doi.org/10.1038/s41419-025-08359-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-025-08359-5