Abstract
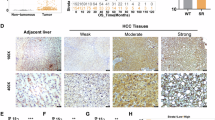
Hepatocellular carcinoma (HCC) is a major global health burden. Despite recent advances in immunotherapy, tyrosine kinase inhibitors (TKIs) treatment or combined therapies, therapeutic resistance and disease progression remain significant challenges. SOX4, a transcription factor frequently overexpressed in HCC and other cancers, has been linked to drug resistance and poor prognosis; however, the underlying molecular mechanisms remain unexplored. In this study, we identify STAT6 as a novel transcriptional target and interacting partner of SOX4 in HCC cells. Genetic ablation or knockdown of SOX4 induced hypermethylation of the STAT6 promoter, suppressing its expression, while treatment with the DNA methyltransferase inhibitor 5-Aza-2’-deoxycytidine restored STAT6 levels, indicating an epigenetic mechanism of regulation. In addition, SOX4 is physically associated with STAT6, as confirmed by co-immunoprecipitation and immunofluorescence. SOX4 depletion impaired interleukin-4 (IL-4)-induced phosphorylation of STAT6 at tyrosine residue 641 (Y641), implicating SOX4 in IL-4-mediated STAT6 activation. Chromatin immunoprecipitation (ChIP) assays demonstrated that SOX4 and STAT6 co-occupy the promoter of MTHFD2, a key enzyme in folate metabolism, regulating NADH/NADPH production and nucleotide biosynthesis. Knockdown of SOX4 or STAT6, or mutation of their binding sites within the MTHFD2 promoter, reduced MTHFD2 expression, NADPH levels, and nucleotide synthesis. Transcriptomic analyses from TCGA-LIHC and our independent cohort revealed a strong positive correlation between SOX4, STAT6, and MTHFD2, with MTHFD2 overexpression linked to poor overall survival. Clinically, elevated SOX4/STAT6/MTHFD2 axis activity was associated with resistance to immunotherapy or TKIs, either in our enrolled cohort or transcriptome data obtained from GSE109211. Metabolomic profiling further revealed increased NADPH and nucleotide biosynthesis in tumors with high SOX4/STAT6/MTHFD2 expression. Targeting STAT6 or MTHFD2 suppressed tumor growth in TKIs-resistant patient-derived xenograft models. Collectively, our findings identify the SOX4–STAT6–MTHFD2 axis as a critical driver of HCC progression and therapeutic resistance, offering a promising target for intervention in refractory HCC.
Similar content being viewed by others
Data availability
All supporting data are provided within the main text and supplementary materials. The high-throughput datasets generated in this study—including DNA methylation, ChIP-seq, and RNA-seq profiles—have been deposited in the GEO under accession numbers GSE286327, GSE277540, and GSE277728, respectively.
References
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63.
Brown ZJ, Tsilimigras DI, Ruff SM, Mohseni A, Kamel IR, Cloyd JM, et al. Management of hepatocellular carcinoma: a review. JAMA Surg. 2023;158:410–20.
Hsu HY, Tang JH, Huang SF, Huang CW, Lin SE, Huang SW, et al. Recurrence pattern is an independent surgical prognostic factor for long-term oncological outcomes in patients with hepatocellular carcinoma. Biomedicines. 2024;12.
Falette Puisieux M, Pellat A, Assaf A, Ginestet C, Brezault C, Dhooge M, et al. Therapeutic management of advanced hepatocellular carcinoma: an updated review. Cancers (Basel). 2022;14.
Haber PK, Puigvehi M, Castet F, Lourdusamy V, Montal R, Tabrizian P, et al. Evidence-based management of hepatocellular carcinoma: systematic review and meta-analysis of randomized controlled trials (2002-2020). Gastroenterology. 2021;161:879–98.
Moreno CS. SOX4: The unappreciated oncogene. Semin Cancer Biol. 2020;67:57–64.
Guan Y, Jiang SR, Liu JG, Shi JR, Liu ZB. USP20 regulates the stability of EMT transcription factor SOX4 and influences colorectal cancer metastasis. Pathol Res Pract. 2022;233:153879.
Li L, Liu J, Xue H, Li C, Liu Q, Zhou Y, et al. A TGF-beta-MTA1-SOX4-EZH2 signaling axis drives epithelial-mesenchymal transition in tumor metastasis. Oncogene. 2020;39:2125–39.
Song GD, Sun Y, Shen H, Li W. SOX4 overexpression is a novel biomarker of malignant status and poor prognosis in breast cancer patients. Tumour Biol. 2015;36:4167–73.
Tiwari N, Tiwari VK, Waldmeier L, Balwierz PJ, Arnold P, Pachkov M, et al. Sox4 is a master regulator of epithelial-mesenchymal transition by controlling Ezh2 expression and epigenetic reprogramming. Cancer Cell. 2013;23:768–83.
Tsai CN, Yu SC, Lee CW, Pang JS, Wu CH, Lin SE, et al. SOX4 activates CXCL12 in hepatocellular carcinoma cells to modulate endothelial cell migration and angiogenesis in vivo. Oncogene. 2020;39:4695–710.
Vervoort SJ, de Jong OG, Roukens MG, Frederiks CL, Vermeulen JF, Lourenco AR, et al. Global transcriptional analysis identifies a novel role for SOX4 in tumor-induced angiogenesis. Elife. 2018;7.
Vervoort SJ, Lourenco AR, Tufegdzic Vidakovic A, Mocholi E, Sandoval JL, Rueda OM, et al. SOX4 can redirect TGF-beta-mediated SMAD3-transcriptional output in a context-dependent manner to promote tumorigenesis. Nucleic Acids Res. 2018;46:9578–90.
Wang D, Hao T, Pan Y, Qian X, Zhou D. Increased expression of SOX4 is a biomarker for. malignant status and poor prognosis in patients with non-small cell lung cancer. Mol Cell Biochem. 2015;402:75–82.
Wang L, Li Y, Yang X, Yuan H, Li X, Qi M, et al. ERG-SOX4 interaction promotes. epithelial-mesenchymal transition in prostate cancer cells. Prostate. 2014;74:647–58.
Wang L, Zhang J, Yang X, Chang YW, Qi M, Zhou Z, et al. SOX4 is associated with poor prognosis in prostate cancer and promotes epithelial-mesenchymal transition in vitro. Prostate Cancer Prostatic Dis. 2013;16:301–7.
Deng X, Wang Y, Guo H, Wang Q, Rao S, Wu H. Pan-cancer analysis and experimental. validation of sox4 as a potential diagnosis, prognosis, and immunotherapy biomarker. Cancers (Basel). 2023;15.
Wu J, Zhu MX, Li KS, Peng L, Zhang PF. Circular RNA drives resistance to anti-PD-1. immunotherapy by regulating the miR-30a-5p/SOX4 axis in non-small cell lung cancer. Cancer Drug Resist. 2022;5:261–70.
Mehta GA, Angus SP, Khella CA, Tong K, Khanna P, Dixon SAH, et al. SOX4 and. SMARCA4 cooperatively regulate PI3k signaling through transcriptional activation of TGFBR2. NPJ Breast Cancer. 2021;7:40.
Maier E, Duschl A, Horejs-Hoeck J. STAT6-dependent and -independent mechanisms in Th2 polarization. Eur J Immunol. 2012;42:2827–33.
Karpathiou G, Papoudou-Bai A, Ferrand E, Dumollard JM, Peoc’h M. STAT6: a review of a signaling pathway implicated in various diseases with a special emphasis in its usefulness in pathology. Pathol Res Pract. 2021;223:153477.
Goenka S, Kaplan MH. Transcriptional regulation by STAT6. Immunol Res. 2011;50:87–96.
Kim EG, Shin HJ, Lee CG, Park HY, Kim YK, Park HW, et al. DNA methylation and not allelic variation regulates STAT6 expression in human T cells. Clin Exp Med. 2010;10:143–52.
Park SJ, Kim H, Kim SH, Joe EH, Jou I. Epigenetic downregulation of STAT6 increases. HIF-1alpha expression via mTOR/S6K/S6, leading to enhanced hypoxic viability of glioma cells. Acta Neuropathol Commun. 2019;7:149.
Binnemars-Postma K, Bansal R, Storm G, Prakash J. Targeting the Stat6 pathway in tumor-associated macrophages reduces tumor growth and metastatic niche formation in breast cancer. FASEB J. 2018;32:969–78.
He K, Barsoumian HB, Puebla-Osorio N, Hu Y, Sezen D, Wasley MD, et al. Inhibition of STAT6 with antisense oligonucleotides enhances the systemic antitumor effects of radiotherapy and anti-PD-1 in metastatic non-small cell lung cancer. Cancer Immunol Res. 2023;11:486–500.
Lee YJ, Kim K, Kim M, Ahn YH, Kang JL. Inhibition of STAT6 Activation by AS1517499. Inhibits expression and activity of PPARgamma in macrophages to resolve acute inflammation in mice. Biomolecules. 2022;12.
Chen J, Gong C, Mao H, Li Z, Fang Z, Chen Q, et al. E2F1/SP3/STAT6 axis is required for. IL-4-induced epithelial-mesenchymal transition of colorectal cancer cells. Int J Oncol. 2018;53:567–78.
Cui X, Zhang L, Luo J, Rajasekaran A, Hazra S, Cacalano N, et al. Unphosphorylated. STAT6 contributes to constitutive cyclooxygenase-2 expression in human non-small cell lung cancer. Oncogene. 2007;26:4253–60.
Fu C, Jiang L, Hao S, Liu Z, Ding S, Zhang W, et al. Activation of the IL-4/STAT6 Signaling Pathway Promotes Lung Cancer Progression by Increasing M2 Myeloid Cells. Front Immunol. 2019;10:2638.
Li BH, Yang XZ, Li PD, Yuan Q, Liu XH, Yuan J, et al. IL-4/Stat6 activities correlate with apoptosis and metastasis in colon cancer cells. Biochem Biophys Res Commun. 2008;369:554–60.
Shi JH, Liu LN, Song DD, Liu WW, Ling C, Wu FX, et al. TRAF3/STAT6 axis regulates macrophage polarization and tumor progression. Cell Death Differ. 2023;30:2005–16.
Wang N, Tao L, Zhong H, Zhao S, Yu Y, Yu B, et al. miR-135b inhibits tumour metastasis in prostate cancer by targeting STAT6. Oncol Lett. 2016;11:543–50.
Qing T, Yamin Z, Guijie W, Yan J, Zhongyang S. STAT6 silencing induces hepatocellular carcinoma-derived cell apoptosis and growth inhibition by decreasing the RANKL expression. Biomed Pharmacother. 2017;92:1–6.
Ducker GS, Rabinowitz JD. One-carbon metabolism in health and disease. Cell Metab. 2017;25:27–42.
Shang M, Yang H, Yang R, Chen T, Fu Y, Li Y, et al. The folate cycle enzyme MTHFD2. induces cancer immune evasion through PD-L1 up-regulation. Nat Commun. 2021;12:1940.
Bonagas N, Gustafsson NMS, Henriksson M, Marttila P, Gustafsson R, Wiita E, et al. Pharmacological targeting of MTHFD2 suppresses acute myeloid leukemia by inducing thymidine depletion and replication stress. Nat Cancer. 2022;3:156–72.
Nishimura T, Nakata A, Chen X, Nishi K, Meguro-Horike M, Sasaki S, et al. Cancer stem-like properties and gefitinib resistance are dependent on purine synthetic metabolism mediated by the mitochondrial enzyme MTHFD2. Oncogene. 2019;38:2464–81.
Wang J, Yu Z, Jiang Y, Le T, Wu Y, Li Z, et al. Downregulation of MTHFD2 Inhibits. Proliferation and Enhances chemosensitivity in hepatocellular carcinoma via PI3K/AKT pathway. Front Biosci (Landmark Ed. 2024;29:35.
Mo HY, Wang RB, Ma MY, Zhang Y, Li XY, Wen WR, et al. MTHFD2-mediated redox. homeostasis promotes gastric cancer progression under hypoxic conditions. Redox Rep. 2024;29:2345455.
Yue L, Pei Y, Zhong L, Yang H, Wang Y, Zhang W, et al. Mthfd2 modulates mitochondrial. function and dna repair to maintain the pluripotency of mouse stem cells. Stem Cell Reports. 2020;15:529–45.
Kamerkar S, Leng C, Burenkova O, Jang SC, McCoy C, Zhang K, et al. Exosome-mediated. genetic reprogramming of tumor-associated macrophages by exoASO-STAT6 leads to potent monotherapy antitumor activity. Sci Adv. 2022;8:eabj7002.
Alhawarri MB. Exploring the anticancer potential of furanpydone a: a computational. study on its inhibition of MTHFD2 across diverse cancer cell lines. Cell Biochem Biophys. 2024;83:437–54.
Li G, Wu J, Li L, Jiang P. p53 deficiency induces MTHFD2 transcription to promote cell. proliferation and restrain DNA damage. Proc Natl Acad Sci USA. 2021;118.
Cagatay T, Ozturk M. P53 mutation as a source of aberrant beta-catenin accumulation in cancer cells. Oncogene. 2002;21:7971–80.
Li M, Sun D, Song N, Chen X, Zhang X, Zheng W, et al. Mutant p53 in head and neck. squamous cell carcinoma: Molecular mechanism of gain‑of‑function and targeting therapy (Review). Oncol Rep. 2023;50.
Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical. strategies. Nat Rev Cancer. 2003;3:330–8.
Kim DW, Talati C, Kim R. Hepatocellular carcinoma (HCC): beyond sorafenib-chemotherapy. J Gastrointest Oncol. 2017;8:256–65.
Verma H, Narendra G, Raju B, Singh PK, Silakari O. Dihydropyrimidine dehydrogenase-mediated resistance to 5-fluorouracil: mechanistic investigation and solution. ACS Pharmacol Transl Sci. 2022;5:1017–33.
Chen JY, Chou HH, Lim SC, Huang YJ, Lai KC, Guo CL, et al. Multiomic characterization. and drug testing establish circulating tumor cells as an ex vivo tool for personalized medicine. iScience. 2022;25:105081.
Broutier L, Mastrogiovanni G, Verstegen MM, Francies HE, Gavarro LM, Bradshaw CR, et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23:1424–35.
Lin RJ, Kuo MW, Yang BC, Tsai HH, Chen K, Huang JR, et al. B3GALT5 knockout alters gycosphingolipid profile and facilitates transition to human naive pluripotency. Proc Natl Acad Sci USA. 2020;117:27435–44.
Lin CY, Wu RC, Huang CY, Lai CH, Chao AS, Li HP, et al. A patient-derived xenograft. model of dedifferentiated endometrial carcinoma: a proof-of-concept study for the identification of new molecularly informed treatment approaches. Cancers (Basel). 2021;13.
Tsai CN, Yu MC, Lee YS, Feng KC, Wu CH, Li YC, et al. NRF2-SOX4 complex regulates. PSPH in hepatocellular carcinoma and modulates M2 macrophage differentiation. Cancer Gene Ther. 2025.
Lin CY, Wu KY, Chi LM, Tang YH, Huang HJ, Lai CH, et al. Starvation-inactivated MTOR. triggers cell migration via a ULK1-SH3PXD2A/TKS5-MMP14 pathway in ovarian carcinoma. Autophagy. 2023;19:3151–68.
Ianevski A, Giri AK, Aittokallio T. SynergyFinder 2.0: visual analytics of multi-drug combination synergies. Nucleic Acids Res. 2020;48:W488–93.
Li HP, Huang CY, Lui KW, Chao YK, Yeh CN, Lee LY, et al. Combination of epithelial. growth factor receptor blockers and CDK4/6 inhibitor for nasopharyngeal carcinoma treatment. Cancers (Basel). 2021;13.
Ma H, Zhang J, Zhou L, Wen S, Tang HY, Jiang B, et al. c-Src promotes tumorigenesis and. tumor progression by activating PFKFB3. Cell Rep. 2020;30:4235–49 e6.
Pang Z, Lu Y, Zhou G, Hui F, Xu L, Viau C, et al. MetaboAnalyst 6.0: towards a unified. platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024;52:W398–406.
Zhu Z, Leung GKK. More than a metabolic enzyme: MTHFD2 as a novel target for. anticancer therapy? Front Oncol. 2020;10:658.
Acknowledgements
The authors deeply appreciated Dr. Jean-Marc Egly (Directeur de Recherche Inserm, Membre de l’Institut-Academie des Sciences) giving critical comments on this manuscript. The authors are also grateful for the valuable support provided by the Microscopy, Genomic Medicine Core Laboratory, Clinical Proteomics Core Laboratory, and Laboratory Animal Center of Chang-Gung Memorial Hospital, Linkou branch. Additionally, they acknowledge the technical assistance of the Metabolomics Core Laboratory of the Healthy Aging Research Center, Chang-Gung University, Taiwan. Special thanks to Miss Yi-Ping Liu for her assistance with data retrieval and processing, as well as to the colleagues from the Research Specimen Processing Laboratory and the New Taipei Municipal Tu-Cheng Hospital for their support. Authors would like to thank Editage (www.editage.com.tw) for English language editing.
Funding
Financial Support by Chang Gung Medical Foundation in Taiwan (CMRPG3M1291/2/3 for CLT; CMRPVVM0093 for MCY, CMRPD1M0611/2 for CNT), and National Science and Technology Council (114-2320-B-182-014 for CNT; 111-2314-B-182A-035-MY3 and 114-2314-B-182A-121 for CLT, 110-2314-B-182A-059-MY3 and 113-2314-B-182A-068 for MCY).
Author information
Authors and Affiliations
Contributions
CL Tsai, MC Yu, and CN Tsai conceptualized and wrote the manuscript. YS Lee conducted the statistical analysis of TCGA-LIHC, ChIP, and RNA sequencing data. Hsu CL developed the HCC-PDx model and performed animal experiments. Chi LM contributed to proteomic processing and data analysis. SE Lin, Hsu HY, and MC Yu facilitated clinical specimen collection and conducted pathological analyses. HY Tang and ML Cheng assisted with LC/MS metabolite analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsai, CL., Yu, MC., Hsu, CL. et al. SOX4-STAT6-MTHFD2 axis drives hepatocellular carcinoma progression and treatment resistance. Cell Death Dis (2026). https://doi.org/10.1038/s41419-025-08394-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-025-08394-2