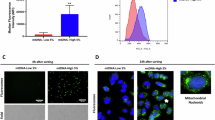
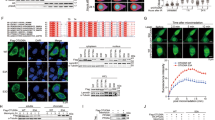
Abstract
Prognosis for pediatric sarcoma (pSC)-affected patients, especially those with relapsed/refractory disease, is dismal. The available treatment options are unsatisfactory, challenging researchers to address this unmet need. The investigational B7-H3 targeted ADC vobramitamab duocarmazine (vobra duo) showed clinical effectiveness towards several B7-H3-positive adult tumors and pre-clinical efficacy in pediatric neuroblastoma models. Cytotoxicity of vobra duo was evaluated in 2D and 3D models toward pSC cell lines expressing B7-H3, showing a dose-dependent cell viability reduction. Proliferation was assessed by time-lapse single-cell segmentation. Compared to controls, vobra duo resulted in a significant increase in the cell doubling time. AKT/mTOR master effectors of cell proliferation were investigated by phospho-specific western blot assays. A down-modulation of phospho-AKT/ -P70 S6K and -4E-BP1 protein expression was detected in both A204 (rhabdomyosarcoma) and U-2-OS (osteosarcoma) cells, the most treatment-sensitive and resistant cell lines, respectively, suggesting their involvement in vobra duo-mediated anti-proliferative effect. In response to treatment, all cell lines underwent apoptotic cell death. A significant increase in the executioner cleaved caspase-3 was detected, and a partial but significant reversion of apoptotic cell death was noted following pre-treatment with the pan-caspase inhibitor, Q-VD-OP-h. Vobra duo also triggered caspase-independent apoptotic events: i) increased AIF nuclear translocation, ii) increased mitochondrial superoxide production, and iii) the depolarization of mitochondrial membrane potential. In vivo, the effectiveness of vobra duo was assayed by single and repeated intravenous administration in the mouse rhabdomyosarcoma model. The single injection of 3 mg/Kg of vobra duo induced a significant tumor growth delay. Repeated vobra duo doses ameliorated this outcome, reverting rhabdomyosarcorma to rhabdomyoma tumor, by increasing Desmin and Myogenin/Myf-4 differentiation markers expression, and reducing both Ki-67 and CD133. In conclusion, the in vitro and in vivo anti-tumor effects towards pSC highlight the need to extend the investigation to patient-derived preclinical models, to pave the way for clinical translation.
Similar content being viewed by others
Data availability
Data are available upon kind request.
References
Williams RF, Fernandez-Pineda I, Gosain A. Pediatric sarcomas. Surg Clin North Am. 2016;96:1107–25.
Hingorani P, Janeway K, Crompton BD, Kadoch C, Mackall CL, Khan J, et al. Current state of pediatric sarcoma biology and opportunities for future discovery: a report from the sarcoma translational research workshop. Cancer Genet. 2016;209:182–94.
Allen-Rhoades W, Whittle SB, Rainusso N. Pediatric solid tumors in children and adolescents: an overview. Pediatrics Rev. 2018;39:444–53.
Anderson JL, Denny CT, Tap WD, Federman N. Pediatric sarcomas: translating molecular pathogenesis of disease to novel therapeutic possibilities. Pediatric Res. 2012;72:112–21.
Pushpam D, Garg V, Ganguly S, Biswas B. Management of refractory pediatric sarcoma: current challenges and future prospects. OncoTargets Therapy. 2020;13:5093–112.
Riccardi F, Dal Bo M, Macor P, Toffoli G. A comprehensive overview on antibody-drug conjugates: from the conceptualization to cancer therapy. Front Pharmacol. 2023;14:1274088.
Grairi M, Le Borgne M. Antibody-drug conjugates: prospects for the next generation. Drug Discov Today. 2024;29:104241.
Dumontet C, Reichert JM, Senter PD, Lambert JM, Beck A. Antibody-drug conjugates come of age in oncology. Nat Reviews Drug Discov. 2023;22:641–61.
Hofmeyer KA, Ray A, Zang X. The contrasting role of B7-H3. Proc Natl Acad Sci USA. 2008;105:10277–8.
Wu S, Hu C, Hui K, Jiang X. Non-immune functions of B7-H3: bridging tumor cells and the tumor vasculature. Front Oncol. 2024;14:1408051.
Feng R, Chen Y, Liu Y, Zhou Q, Zhang W. The role of B7-H3 in tumors and its potential in clinical application. Int Immunopharmacol. 2021;101:108153.
Picarda E, Ohaegbulam KC, Zang X. Molecular pathways: targeting B7-H3 (CD276) for human cancer immunotherapy. Clin Cancer Res. 2016;22:3425–31.
Pulido R, López JI, Nunes-Xavier CE. B7-H3: a robust target for immunotherapy in prostate cancer. Trend Cancer. 2024;10:584–7.
Liu C, Zhang G, Xiang K, Kim Y, Lavoie RR, Lucien F, et al. Targeting the immune checkpoint B7-H3 for next-generation cancer immunotherapy. Cancer Immunol Immunother CII. 2022;71:1549–67.
Epperly R, Gottschalk S, DeRenzo C. CAR T cells redirected to B7-H3 for pediatric solid tumors: current status and future perspectives. EJC Paediatric Oncol. 2024;3:100160.
Feustel K, Martin J, Falchook GS. B7-H3 inhibitors in oncology clinical trials: a review. J Immunother Precision Oncol. 2024;7:53–66.
Jang S, Powderly JD, Spira AI, Bakkacha O, Loo D, Bohac GC, et al. Phase 1 dose escalation study of MGC018, an anti-B7-H3 antibody-drug conjugate (ADC), in patients with advanced solid tumors. Journal of Clinical Oncology. 2021;39:2631.
Shenderov E, Mallesara GHG, Wysocki PJ, Xu W, Ramlau R, Weickhardt AJ, et al. 620P MGC018, an anti-B7-H3 antibody-drug conjugate (ADC), in patients with advanced solid tumors: Preliminary results of phase I cohort expansion. Annal Oncol. 2021;32:S657–S9.
Brignole C, Calarco E, Bensa V, Giusto E, Perri P, Ciampi E, et al. Antitumor activity of the investigational B7-H3 antibody-drug conjugate, vobramitamab duocarmazine, in preclinical models of neuroblastoma. J Immunother Cancer. 2023;11:e007174.
Kurmasheva R, Mosse Y, Pozo V, Earley E, Erickson S, Groff D, et al. Testing of B7-H3 targeting antibody-drug conjugate (ADC) MGC018 in models of pediatric solid tumors by the Pediatric Preclinical Testing Consortium (PPTC). J Clin Oncol. 2021;39:10037.
Chen S, Ma J, Yang L, Teng M, Lai Z-Q, Chen X, et al. Anti-glioblastoma activity of kaempferol via programmed cell death induction: involvement of autophagy and pyroptosis. Front Bioeng Biotechnol. 2020;8:614419.
Bertola N, Regis S, Cossu V, Balbi M, Serra M, Corsolini F, et al. miR-29a-3p and TGF-β Axis in Fanconi anemia: mechanisms driving metabolic dysfunction and genome stability. Cellular and molecular life sciences : CMLS. 2025;82:255.
Ravera S, Vigliarolo T, Bruno S, Morandi F, Marimpietri D, Sabatini F, et al. Identification of biochemical and molecular markers of early aging in childhood cancer survivors. Cancers. 2021;13:5214.
Monteleone L, Speciale A, Valenti GE, Traverso N, Ravera S, Garbarino O, et al. PKCα inhibition as a strategy to sensitize neuroblastoma stem cells to etoposide by stimulating ferroptosis. Antioxidants. 2021;10:691.
Liu C, Liu S, Wang S, Sun Y, Lu X, Li H, et al. IGF-1 Via PI3K/Akt/S6K signaling pathway protects DRG neurons with high glucose-induced toxicity. Open Life Sci. 2019;14:502–14.
Matheny RW Jr., Adamo ML. Role of Akt isoforms in IGF-I-mediated signaling and survival in myoblasts. Biochem Biophys Res Commun. 2009;389:117–21.
Scribner JA, Brown JG, Son T, Chiechi M, Li P, Sharma S, et al. Preclinical development of MGC018, a duocarmycin-based antibody-drug conjugate targeting B7-H3 for solid cancer. Mol Cancer Therap. 2020;19:2235–44.
Xu J, Timares L, Heilpern C, Weng Z, Li C, Xu H, et al. Targeting wild-type and mutant p53 with small molecule CP-31398 blocks the growth of rhabdomyosarcoma by inducing reactive oxygen species-dependent apoptosis. Cancer Res. 2010;70:6566–76.
Bai Y, Li J, Fang B, Edwards A, Zhang G, Bui M, et al. Phosphoproteomics identifies driver tyrosine kinases in sarcoma cell lines and tumors. Cancer Res. 2012;72:2501–11.
Novo N, Romero-Tamayo S, Marcuello C, Boneta S, Blasco-Machin I, Velázquez-Campoy A, et al. Beyond a platform protein for the degradosome assembly: the apoptosis-inducing factor as an efficient nuclease involved in chromatinolysis. PNAS Nexus. 2022;2:pgac312.
Sevrioukova IF. Apoptosis-inducing factor: structure, function, and redox regulation. Antioxid Redox Signal. 2011;14:2545–79.
Hernandez Tejada FN, Zamudio A, Marques-Piubelli ML, Cuglievan B, Harrison D. Advances in the management of pediatric sarcomas. Curr Oncol Rep. 2020;23:3.
Dyson KA, Stover BD, Grippin A, Mendez-Gomez HR, Lagmay J, Mitchell DA, et al. Emerging trends in immunotherapy for pediatric sarcomas. J Hematol Oncol. 2019;12:78.
Lambert JM, Morris CQ. Antibody–drug conjugates (ADCs) for personalized treatment of solid tumors: a review. Adv Therapy. 2017;34:1015–35.
Stokke JL, Bhojwani D. Antibody-drug conjugates for the treatment of acute pediatric leukemia. J Clin Med. 2021;10:3556.
Hingorani P, Zhang W, Zhang Z, Xu Z, Wang WL, Roth ME, et al. Trastuzumab deruxtecan, antibody-drug conjugate targeting HER2, is effective in pediatric malignancies: a report by the pediatric preclinical testing consortium. Mol Cancer Therap. 2022;21:1318–25.
Bottino C, Vitale C, Dondero A, Castriconi R. B7-H3 in pediatric tumors: far beyond neuroblastoma. Cancers. 2023;15:3279.
Kendsersky NM, Lindsay J, Kolb EA, Smith MA, Teicher BA, Erickson SW, et al. The B7-H3-targeting antibody-drug conjugate m276-SL-PBD is potently effective against pediatric cancer preclinical solid tumor models. Clin Cancer Res. 2021;27:2938–46.
Kurmasheva R, Mosse YP, Pozo VD, Earley EJ, Erickson SW, Groff D, et al. Testing of B7-H3 targeting antibody-drug conjugate (ADC) MGC018 in models of pediatric solid tumors by the Pediatric Preclinical Testing Consortium (PPTC). J Clin Oncol. 2021;39:10037.
Smith MA, Houghton PJ, Lock RB, Maris JM, Gorlick R, Kurmasheva RT, et al. Lessons learned from 20 years of preclinical testing in pediatric cancers. Pharmacol Therap. 2024;264:108742.
Boshuizen J, Peeper DS. Rational cancer treatment combinations: an urgent clinical need. Mol cell. 2020;78:1002–18.
Yamato M, Hasegawa J, Maejima T, Hattori C, Kumagai K, Watanabe A, et al. DS-7300a, a DNA topoisomerase I inhibitor, DXd-based antibody-drug conjugate targeting B7-H3, exerts potent Antitumor activities in preclinical models. Mol Cancer Therap. 2022;21:635–46.
Wang J, Duan J, Sun Y, Xing L, Han L, Wang Q, et al. ARTEMIS-001: Data from a phase 1a/b study of HS-20093 in patients with relapsed small cell lung cancer (SCLC). J Clin Oncol. 2024;42:8093.
De Luca A, Raimondi L, Salamanna F, Carina V, Costa V, Bellavia D, et al. Relevance of 3d culture systems to study osteosarcoma environment. J Exp clin Cancer Res CR. 2018;37:2.
Ravi M, Paramesh V, Kaviya SR, Anuradha E, Solomon FD. 3D cell culture systems: advantages and applications. J Cell Physiol. 2015;230:16–26.
Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501.
Peng Y, Wang Y, Zhou C, Mei W, Zeng C. PI3K/Akt/mTOR pathway and its role in cancer therapeutics: are we making headway?. Front Oncol. 2022;12:819128.
Versari I, Salucci S, Bavelloni A, Battistelli M, Traversari M, Wang A, et al. The emerging role and clinical significance of PI3K-Akt-mTOR in rhabdomyosarcoma. Biomolecules. 2025;15:334.
Zhang J, Yu X-H, Yan Y-G, Wang C, Wang W-J. PI3K/Akt signaling in osteosarcoma. Clinica Chimica Acta. 2015;444:182–92.
Toson B, Fortes IS, Roesler R, Andrade SF. Targeting Akt/PKB in pediatric tumors: a review from preclinical to clinical trials. Pharmacol Res. 2022;183:106403.
Damerell V, Pepper MS, Prince S. Molecular mechanisms underpinning sarcomas and implications for current and future therapy. Signal Transduct Targeted Ther. 2021;6:246.
Yung HW, Charnock-Jones DS, Burton GJ. Regulation of AKT phosphorylation at Ser473 and Thr308 by endoplasmic reticulum stress modulates substrate specificity in a severity dependent manner. PLoS ONE. 2011;6:e17894.
Heberle AM, Prentzell MT, van Eunen K, Bakker BM, Grellscheid SN, Thedieck K. Molecular mechanisms of mTOR regulation by stress. Mol Cell Oncol. 2015;2:e970489.
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46.
Boland ML, Chourasia AH, Macleod KF. Mitochondrial dysfunction in cancer. Front Oncol. 2013;3:292.
Miallot R, Galland F, Millet V, Blay J-Y, Naquet P. Metabolic landscapes in sarcomas. J Hematol Oncol. 2021;14:114.
Wang Y, Qi H, Liu Y, Duan C, Liu X, Xia T, et al. The double-edged roles of ROS in cancer prevention and therapy. Theranostics. 2021;11:4839–57.
Bano D, Prehn JHM. Apoptosis-inducing factor (AIF) in physiology and disease: the tale of a repented natural born killer. eBioMedicine. 2018;30:29–37.
Toki S, Yoshimaru T, Matsushita Y, Aihara H, Ono M, Tsuneyama K, et al. The survival and proliferation of osteosarcoma cells are dependent on the mitochondrial BIG3-PHB2 complex formation. Cancer Sci. 2021;112:4208–19.
Xu C, Huang X, Hu Q, Xue W, Zhou K, Li X, et al. Modulating autophagy to boost the antitumor efficacy of TROP2-directed antibody-drug conjugate in pancreatic cancer. Biomed Pharmacother Biomed Pharmacotherapie. 2024;180:117550.
Yan H, Endo Y, Shen Y, Rotstein D, Dokmanovic M, Mohan N, et al. Ado-trastuzumab emtansine targets hepatocytes via human epidermal growth factor receptor 2 to induce hepatotoxicity. Mol Cancer Therap. 2016;15:480–90.
Sroka MW, Skopelitis D, Vermunt MW, Preall JB, El Demerdash O, de Almeida LMN, et al. Myo-differentiation reporter screen reveals NF-Y as an activator of PAX3–FOXO1 in rhabdomyosarcoma. Proc Natl Acad Sci USA. 2023;120:e2303859120.
Godbole P, Outram A, Wilcox DT, Duffy PG, Sebire NJ. Myogenin and desmin immunohistochemistry in the assessment of post-chemotherapy genitourinary embryonal rhabdomyosarcoma: prognostic and management implications. J Urol. 2006;176:1751–4.
Keller C, Guttridge DC. Mechanisms of impaired differentiation in rhabdomyosarcoma. FEBS J. 2013;280:4323–34.
Pomella S, Danielli SG, Alaggio R, Breunis WB, Hamed E, Selfe J, et al. Genomic and epigenetic changes drive aberrant skeletal muscle differentiation in rhabdomyosarcoma. Cancers. 2023;15:2823.
Yang Z, MacQuarrie KL, Analau E, Tyler AE, Dilworth FJ, Cao Y, et al. MyoD and E-protein heterodimers switch rhabdomyosarcoma cells from an arrested myoblast phase to a differentiated state. Genes Dev. 2009;23:694–707.
Hüttner SS, Henze H, Elster D, Koch P, Anderer U, von Eyss B, et al. A dysfunctional miR-1-TRPS1-MYOG axis drives ERMS by suppressing terminal myogenic differentiation. Mol Ther. 2023;31:2612–32.
Miekus K, Lukasiewicz E, Jarocha D, Sekula M, Drabik G, Majka M. The decreased metastatic potential of rhabdomyosarcoma cells obtained through MET receptor downregulation and the induction of differentiation. Cell Death Dis. 2013;4:e459.
Walter D, Satheesha S, Albrecht P, Bornhauser BC, D’Alessandro V, Oesch SM, et al. CD133 positive embryonal rhabdomyosarcoma stem-like cell population is enriched in rhabdospheres. PLoS ONE. 2011;6:e19506.
Radzikowska J, Czarnecka AM, Klepacka T, Rychłowska-Pruszyńska M, Raciborska A, Dembowska-Bagińska B, et al. Cancer stem cell markers in rhabdomyosarcoma in children. Diagnostics. 2022;12:1895.
Takenobu H, Shimozato O, Nakamura T, Ochiai H, Yamaguchi Y, Ohira M, et al. CD133 suppresses neuroblastoma cell differentiation via signal pathway modification. Oncogene. 2011;30:97–105.
Acknowledgements
The Authors thanks Macrogenics Inc. (Rockville, MD, USA) for providing vobra duo.
Funding
This work was supported by Italian Ministry of Health (“Ricerca Corrente” to P.M. and to Scientific Direction, IRCCS Istituto Giannina Gaslini) and by Fondazione Italiana per la Lotta al Neuroblastoma. E.C. is a recipient of AIRC ID.24397 contract. G.R. is a recipient of a Fondazione Italiana per la Lotta al Neuroblastoma fellowship.
Author information
Authors and Affiliations
Contributions
G.B., C.B. study conceptualization, experimental design, experimental work, and data analysis. G.R., E.C., D.S., S.R., M.B., experimental work and data analyses. F.P. experimental work, experimental design, and data analyses. B. DG. histological and immunehistochemical staining. B.C., V.G.V. histological and immunehistochemical analyses. G.B., C.B., S.R., V.G.V., manuscript preparation and revision. M.P. supervision of the project, data analyses. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
Authors declare no conflict of interest
Ethics approval and consent to participate
All methods were performed in accordance with the relevant institutional and national guidelines and regulations. Animal experiments were approved by the Animal Welfare Body (OPBA) of IRCCS Ospedale Policlinico San Martino and by the Italian Ministry of Health (approval number: 539/2024-PR). No human participant were involved in this research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bianchi, G., Pastorino, F., Rolandi, G. et al. The investigational anti-B7-H3 antibody-drug conjugate vobramitamab duocarmazine exerts anti-tumor activity in vitro and in vivo in pediatric sarcoma preclinical models. Cell Death Dis (2026). https://doi.org/10.1038/s41419-025-08397-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-025-08397-z