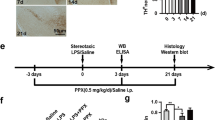
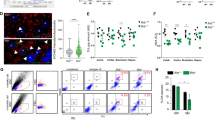
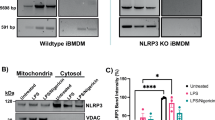
Abstract
Dysregulated mitochondrial DNA (mtDNA) promotes inflammatory response and disease progression. However, the mechanism and role of mtDNA-mediated inflammatory activation in the pathogenesis of Parkinson’s disease (PD) are not yet clear. This study demonstrates that the injection of mtDNA into the substantia nigra pars compacta induces PD pathology in mice, characterized by the loss of dopaminergic (DA) neurons and the activation of microglia. Transcriptomic profiling of magnetic-activated cell sorting (MACS)-sorted cells reveals a pronounced upregulation of genes associated with the NLRP3 inflammasome pathway in microglia following the mtDNA administration. Critically, lipopolysaccharide (LPS) and rotenone induced in vivo and in vitro PD models show oxidized mtDNA (ox-mtDNA) release and microglial NLRP3-IL-1β axis activation as evidenced by upregulation of NLRP3 and IL-1β, caspase-1 cleavage, and IL-1β release. The role of mtDNA in activating the NLRP3-IL-1β axis is further validated in BV2 cells through exogeneous mtDNA transfection, while the NLRP3-IL-1β activation is negated in the LPS and rotenone induced model when mtDNA release is inhibited. Especially, oxidized mtDNA is superior to nonoxidized mtDNA in activating the NLRP3-IL-1β axis. NLRP3 knockdown in BV2 cells abolishes the activation of NLRP3-IL-1β axis induced by mtDNA or exposure of LPS and rotenone and mitigates the damage to SH-SY5Y cells in co-culture systems. Ox-mtDNA-mediated neuronal cell damage is initiated through binding to NLRP3, as demonstrated by co-immunoprecipitation and co-localization in BV2 cells. Molecular docking prediction and analysis of intrinsically disordered region (IDR) of NLRP3 indicate that ox-mtDNA interacts with the positively charged IDR of NLRP3. This interaction is validated by electrophoretic mobility shift and in vitro PYD-caspase-1 cleavage assays, demonstrating the formation of the ox-mtDNA-NLRP3 complex and subsequent activation of NLRP3. This study describes a critical role of mtDNA in activating microglial NLRP3-IL-1β axis, leading to neurodegeneration in PD pathology, which provides clear clues for developing anti-PD drugs targeting NLRP3.
Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author [szhou@zmu.edu.cn] upon reasonable request.
References
Tolosa E, Garrido A, Scholz SW, Poewe W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021;20:385–97.
Zhang D, Li S, Hou L, Jing L, Ruan Z, Peng B, et al. Microglial activation contributes to cognitive impairments in rotenone-induced mouse Parkinson’s disease model. J Neuroinflammation. 2021;18:4.
Zhao W, Liu Z, Wu J, Liu A, Yan J. Potential targets of microglia in the treatment of neurodegenerative diseases: mechanism and therapeutic implications. Neural Regen Res. 2026;21:1497–511.
Soraci L, Gambuzza ME, Biscetti L, Laganà P, Lo Russo C, Buda A, et al. Toll-like receptors and NLRP3 inflammasome-dependent pathways in Parkinson’s disease: mechanisms and therapeutic implications. J Neurol. 2023;270:1346–60.
Gu YY, Zhao XR, Zhang N, Yang Y, Yi Y, Shao QH, et al. Mitochondrial dysfunction as a therapeutic strategy for neurodegenerative diseases: current insights and future directions. Ageing Res Rev. 2024;102:102577.
Wright R. Mitochondrial dysfunction and Parkinson’s disease. Nat Neurosci. 2022;25:2.
Flønes IH, Toker L, Sandnes DA, Castelli M, Mostafavi S, Lura N, et al. Mitochondrial complex I deficiency stratifies idiopathic Parkinson’s disease. Nat Commun. 2024;15:3631.
Takeuchi H, Mizuno T, Zhang G, Wang J, Kawanokuchi J, Kuno R, et al. Neuritic beading induced by activated microglia is an early feature of neuronal dysfunction toward neuronal death by inhibition of mitochondrial respiration and axonal transport. J Biol Chem. 2005;280:10444–54.
Tresse E, Marturia-Navarro J, Sew WQG, Cisquella-Serra M, Jaberi E, Riera-Ponsati L, et al. Mitochondrial DNA damage triggers spread of Parkinson’s disease-like pathology. Mol Psychiatry. 2023;28:4902–14.
West AP, Shadel GS. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat Rev Immunol. 2017;17:363–75.
Marchi S, Guilbaud E, Tait SWG, Yamazaki T, Galluzzi L. Mitochondrial control of inflammation. Nat Rev Immunol. 2023;23:159–73.
Maatouk L, Compagnion A-C, Sauvage M-AC-d, Bemelmans A-P, Leclere-Turbant S, Cirotteau V, et al. TLR9 activation via microglial glucocorticoid receptors contributes to degeneration of midbrain dopamine neurons. Nat Commun. 2018;9:2450.
Gulen MF, Samson N, Keller A, Schwabenland M, Liu C, Glück S, et al. cGAS–STING drives ageing-related inflammation and neurodegeneration. Nature. 2023;620:374–80.
Zhong Z, Liang S, Sanchez-Lopez E, He F, Shalapour S, Lin XJ, et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature. 2018;560:198–203.
Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–14.
Haque ME, Akther M, Jakaria M, Kim IS, Azam S, Choi DK. Targeting the microglial NLRP3 inflammasome and its role in Parkinson’s disease. Mov Disord. 2020;35:20–33.
Yan YQ, Zheng R, Liu Y, Ruan Y, Lin ZH, Xue NJ, et al. Parkin regulates microglial NLRP3 and represses neurodegeneration in Parkinson’s disease. Aging cell. 2023;22:e13834.
Szeto HH, Liu S, Soong Y, Seshan SV, Cohen-Gould L, Manichev V, et al. Mitochondria protection after acute ischemia prevents prolonged upregulation of IL-1β and IL-18 and arrests CKD. J Am Soc Nephrol. 2017;28:1437–49.
Wu Y, Hao C, Liu X, Han G, Yin J, Zou Z, et al. MitoQ protects against liver injury induced by severe burn plus delayed resuscitation by suppressing the mtDNA-NLRP3 axis. Int Immunopharmacol. 2020;80:106189.
Xu W, Huang Y, Zhou R. NLRP3 inflammasome in neuroinflammation and central nervous system diseases. Cell Mol Immunol. 2025;22:341–55.
Li T, Tan X, Tian L, Jia C, Cheng C, Chen X, et al. The role of Nurr1-miR-30e-5p-NLRP3 axis in inflammation-mediated neurodegeneration: insights from mouse models and patients’ studies in Parkinson’s disease. J Neuroinflammation. 2023;20:274.
Ou Z, Zhou Y, Wang L, Xue L, Zheng J, Chen L, et al. NLRP3 inflammasome inhibition prevents α-synuclein pathology by relieving autophagy dysfunction in chronic MPTP-treated NLRP3 knockout mice. Mol Neurobiol. 2021;58:1303–11.
Kong L, Li W, Chang E, Wang W, Shen N, Xu X, et al. mtDNA-STING axis mediates microglial polarization via IRF3/NF-κB signaling after ischemic stroke. Front Immunol. 2022;13:860977.
Guan X, Zhu S, Song J, Liu K, Liu M, Xie L, et al. Microglial CMPK2 promotes neuroinflammation and brain injury after ischemic stroke. Cell Rep Med. 2024;5:101522.
Liang Z, Damianou A, Vendrell I, Jenkins E, Lassen FH, Washer SJ, et al. Proximity proteomics reveals UCH-L1 as an essential regulator of NLRP3-mediated IL-1β production in human macrophages and microglia. Cell Rep. 2024;43:114152.
Morton KS, George AJ, Meyer JN. Complex I superoxide anion production is necessary and sufficient for complex I inhibitor-induced dopaminergic neurodegeneration in Caenorhabditis elegans. Redox Biol. 2025;81:103538.
Norat P, Soldozy S, Sokolowski JD, Gorick CM, Kumar JS, Chae Y, et al. Mitochondrial dysfunction in neurological disorders: exploring mitochondrial transplantation. NPJ Regen Med. 2020;5:22.
Pérez-Treviño P, Velásquez M, García N. Mechanisms of mitochondrial DNA escape and its relationship with different metabolic diseases. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165761.
Roy T, Chatterjee A, Swarnakar S. Rotenone induced neurodegeneration is mediated via cytoskeleton degradation and necroptosis. Biochim Biophys Acta Mol Cell Res. 2023;1870:119417.
Ravanat JL, Di Mascio P, Martinez GR, Medeiros MH, Cadet J. Singlet oxygen induces oxidation of cellular DNA. J Biol Chem. 2000;275:40601–4.
Alexeyev M, Shokolenko I, Wilson G, LeDoux S. The maintenance of mitochondrial DNA integrity-critical analysis and update. Cold Spring Harb Perspect Biol. 2013;5:a012641.
Marques E, Kramer R, Ryan DG. Multifaceted mitochondria in innate immunity. NPJ Metab Health Dis. 2024;2:6.
Jonas F, Navon Y, Barkai N. Intrinsically disordered regions as facilitators of the transcription factor target search. Nat Rev Genet. 2025;26:424–35.
Guo B, Gu J, Zhuang T, Zhang J, Fan C, Li Y, et al. MicroRNA-126: from biology to therapeutics. Biomed Pharmacother. 2025;185:117953.
Sarkar S, Malovic E, Harishchandra DS, Ghaisas S, Panicker N, Charli A, et al. Mitochondrial impairment in microglia amplifies NLRP3 inflammasome proinflammatory signaling in cell culture and animal models of Parkinson’s disease. NPJ Parkinsons Dis. 2017;3:30.
Jankovic J, Tan EK. Parkinson’s disease: etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry. 2020;91:795–808.
Ni J, Wu Z, Meng J, Saito T, Saido TC, Qing H, et al. An impaired intrinsic microglial clock system induces neuroinflammatory alterations in the early stage of amyloid precursor protein knock-in mouse brain. J Neuroinflammation. 2019;16:173.
Lawana V, Singh N, Sarkar S, Charli A, Jin H, Anantharam V, et al. Involvement of c-Abl kinase in microglial activation of NLRP3 inflammasome and impairment in autolysosomal system. J Neuroimmune Pharmacol. 2017;12:624–60.
Li T, Li Y, Chen J, Nan M, Zhou X, Yang L, et al. Hyperibone J exerts antidepressant effects by targeting ADK to inhibit microglial P2X7R/TLR4-mediated neuroinflammation. J Adv Res. 2025;72:571–89.
Pan-Montojo F, Anichtchik O, Dening Y, Knells L, Pursche S, Jung R, et al. Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS ONE. 2010;5:e8762.
Pfeifer GP. DNA damage and Parkinson’s disease. Int J Mol Sci. 2024;25:4187.
Cao B, Wang T, Qu Q, Kang T, Yang Q. Long noncoding RNA SNHG1 promotes neuroinflammation in Parkinson’s disease via regulating miR-7/NLRP3 pathway. Neuroscience. 2018;388:118–27.
Zhang W, Li G, Luo R, Lei J, Song Y, Wang B, et al. Cytosolic escape of mitochondrial DNA triggers cGAS-STING-NLRP3 axis-dependent nucleus pulposus cell pyroptosis. Exp Mol Med. 2022;54:129–42.
Pan J, Ou Z, Cai C, Li P, Gong J, Ruan XZ, et al. Fatty acid activates NLRP3 inflammasomes in mouse Kupffer cells through mitochondrial DNA release. Cell Immunol. 2018;332:111–20.
Cabral A, Cabral JE, Wang A, Zhang Y, Liang H, Nikbakht D, et al. Differential binding of NLRP3 to non-oxidized and Ox-mtDNA mediates NLRP3 inflammasome activation. Commun Biol. 2023;6:578.
Zou G, Tang Y, Yang J, Fu S, Li Y, Ren X, et al. Signal-induced NLRP3 phase separation initiates inflammasome activation. Cell Res. 2025;35:437–52.
Zhang Q, Zhou J, Shen M, Xu H, Yu S, Cheng Q, et al. Pyrroloquinoline quinone inhibits rotenone-induced microglia inflammation by enhancing autophagy. Molecules. 2020;25:4359.
Shen Y, Wang X, Nan N, Fu X, Zeng R, Yang Y, et al. SIRT3-mediated deacetylation of SDHA rescues mitochondrial bioenergetics contributing to neuroprotection in rotenone-induced PD models. Mol Neurobiol. 2024;61:4402–20.
Zhang W, Zhang M, Wu Q, Shi J. Dendrobium nobile Lindl. Alkaloids ameliorate Aβ25-35-induced synaptic deficits by targeting Wnt/β-catenin pathway in Alzheimer’s disease models. J Alzheimers Dis. 2022;86:297–313.
Bryant JD, Lei Y, VanPortfliet JJ, Winters AD, West AP. Assessing mitochondrial DNA release into the cytosol and subsequent activation of innate immune-related pathways in mammalian cells. Curr Protoc. 2022;2:e372.
Jiménez-Loygorri JI, Villarejo-Zori B, Viedma-Poyatos Á, Zapata-Muñoz J, Benítez-Fernández R, Frutos-Lisón MD, et al. Mitophagy curtails cytosolic mtDNA-dependent activation of cGAS/STING inflammation during aging. Nat Commun. 2024;15:830.
Wang Y, Fu X, Zeng L, Hu Y, Gao R, Xian S, et al. Activation of Nrf2/HO-1 signaling pathway exacerbates cholestatic liver injury. Commun Biol. 2024;7:621.
Acknowledgements
We express our gratitude for the experimental platform and technical support provided by Zunyi Medical University.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 82260806), Guizhou Provincial Science and Technology Project (Qiankehe foundation-ZK [2024] general 274) and Zunyi Science and Technology Bureau Project (Zunshikehe -HZ [2022] 422).
Author information
Authors and Affiliations
Contributions
SZ, QG and XF designed all aspects of the study; NN and YY designed some methods of the study; YW performed transcriptome sequencing analysis; QG, TZ, NF, SG, and LH performed experiments and analysis; SZ and XF provided critical support for manuscript and supervision; SZ, QG and TZ wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
ETHICS
The animal protocols were approved by the Experimental Animal Ethics Committee of the Zunyi Medical University (No. ZMU 21-2303-332) and were in line with the International Guidelines for Care and Use of Laboratory Animals (National Academy of Sciences Health Publication No. 85-23, revised in 1996).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Bertrand Joseph
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gan, Q., Fu, X., Zhou, T. et al. Mitochondrial DNA drives NLRP3-IL-1β axis activation in microglia by binding to NLRP3, leading to neurodegeneration in Parkinson’s disease models. Cell Death Dis (2026). https://doi.org/10.1038/s41419-026-08424-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-026-08424-7