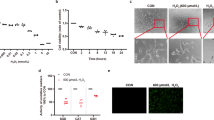
Abstract
Photoreceptors (PRs) are specialized light-sensitive cells responsible for vision, and their death is the primary cause of retinal degeneration and vision loss. Recent studies using cells such as HeLa and PC12 have demonstrated cellular recovery even from late stages of apoptosis. Here, we demonstrate for the first time that PR cells can recover from features of apoptosis following exposure to apoptotic stressors. Upon apoptotic stimuli (staurosporine or hypoxia), 661 W cells, a murine cone PR cell line, exhibited morphological and functional features of apoptosis, such as rounding and blebbing, caspase-3 activation, PARP cleavage, and phosphatidylserine externalization. These processes were reversed upon the alleviation of stress. We also observed that mitochondrial function is central to apoptotic recovery of photoreceptor cells, as evidenced by the restoration of intracellular ATP levels and reduction in mitochondrial reactive oxygen species (mROS). Mitophagy was demonstrated to play a crucial role in cell survival, with increased protein and mRNA expression of mitophagy markers during recovery from apoptosis. Furthermore, the modulation of mitophagy confirmed its protective role in the recovery phase, as its induction with MF-094 reduced apoptosis while its inhibition with Mdivi-1 exacerbated cell death. In vivo, we demonstrate the recovery of PRs from apoptosis using an experimental model of transient retinal detachment. Altogether, the findings of this study indicate that PR cells can recover from entry into the apoptotic cascade, and that mitophagy is essential for apoptotic recovery in these cells.
Similar content being viewed by others
Data availability
All data generated and analyzed in this study are presented in this published article. Primary data may be made available from the corresponding author upon reasonable request.
References
Tonade D, Kern T. Photoreceptor cells and RPE contribute to the development of diabetic retinopathy. Prog Retin Eye Res. 2021;83:100919.
Murakami Y, Notomi S, Hisatomi T, Nakazawa T, Ishibashi T, Miller J, et al. Photoreceptor cell death and rescue in retinal detachment and degenerations. Prog Retin Eye Res. 2013;37:114–40.
Dunaief J, Dentchev T, Ying G, Milam A. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002;120:1435–42.
Besirli C, Chinskey N, Zheng Q, Zacks D. Inhibition of retinal detachment-induced apoptosis in photoreceptors by a small peptide inhibitor of the fas receptor. Invest Ophthalmol Vis Sci. 2010;51:2177–84.
Sanges D, Comitato A, Tammaro R, Marigo V. Apoptosis in retinal degeneration involves cross-talk between apoptosis-inducing factor (AIF) and caspase-12 and is blocked by calpain inhibitors. Proc Natl Acad Sci USA. 2006;103:17366–71.
Guérin C, Lewis G, Fisher S, Anderson D. Recovery of photoreceptor outer segment length and analysis of membrane assembly rates in regenerating primate photoreceptor outer segments. Invest Ophthalmol Vis Sci. 1993;34:175–83.
Kaplan M, Iwata R, Sterrett C. Retinal detachment prevents normal assembly of disk membranes in vitro. Invest Ophthalmol Vis Sci. 1990;31:1–8.
Annan W, Asamani E, White D Mathematical model for rod outer segment dynamics during retinal detachment. PLoS ONE. 2024;19:e0297419.
Rex T, Fariss R, Lewis G, Linberg K, Sokal I, Fisher S. A survey of molecular expression by photoreceptors after experimental retinal detachment. Invest Ophthalmol Vis Sci. 2002;43:1234–47.
Lewis G, Matsumoto B, Fisher S. Changes in the organization and expression of cytoskeletal proteins during retinal degeneration induced by retinal detachment. Invest Ophthalmol Vis Sci. 1995;36:2404–16.
Abcouwer SF, Scavuzzi BM, Kish PE, Kong D, Shanmugam S, Le XA, et al. The mouse retinal pigment epithelium mounts an innate immune defense response following retinal detachment. J Neuroinflammat. 2024;21:74.
Zacks D, Han Y, Zeng Y, Swaroop A. Activation of signaling pathways and stress-response genes in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 2006;47:1691–5.
Zacks D, Hänninen V, Pantcheva M, Ezra E, Grosskreutz C, Miller J. Caspase activation in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 2003;44:1262–7.
Besirli C, Chinskey N, Zheng Q, Zacks D. Autophagy activation in the injured photoreceptor inhibits fas-mediated apoptosis. Invest Ophthalmol Vis Sci. 2011;52:4193–9.
Zacks D. Gene transcription profile of the detached retina (An AOS Thesis). Trans Am Ophthalmol Soc. 2009;107:343–82.
Kiang L, Ross B, Yao J, Shanmugam S, Andrews C, Hansen S, et al. Vitreous cytokine expression and a murine model suggest a key role of microglia in the inflammatory response to retinal detachment. Invest Ophthalmol Vis Sci. 2018;59:3767–78.
Cook B, Lewis G, Fisher S, Adler R. Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest Ophthalmol Vis Sci. 1995;36:990–6.
Hisatomi T, Sakamoto T, Murata T, Yamanaka I, Oshima Y, Hata Y, et al. Relocalization of apoptosis-inducing factor in photoreceptor apoptosis induced by retinal detachment in vivo. Am J Pathol. 2001;158:1271–8.
Yang L, Bula D, Arroyo J, Chen D. Preventing retinal detachment-associated photoreceptor cell loss in Bax-deficient mice. Invest Ophthalmol Vis Sci. 2004;45:648–54.
You W, Knoops K, Boesten I, Berendschot T, van Zandvoort M, Benedikter B, et al. A time window for rescuing dying retinal ganglion cells. Cell Commun Signal. 2024;22:88.
Tang H, Tang H, Mak K, Hu S, Wang S, Wong K, et al. Cell survival, DNA damage, and oncogenic transformation after a transient and reversible apoptotic response. Mol Biol Cell. 2012;23:2240–52.
Tang H, Tang H Anastasis: recovery from the brink of cell death. R Soc Open Sci. 2018;5:180442.
Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516.
Hoeppner D, Hengartner M, Schnabel R. Engulfment genes cooperate with ced-3 to promote cell death in Caenorhabditis elegans. Nature. 2001;412:202–6.
Van Opdenbosch N, Lamkanfi M. Caspases in Cell Death, Inflammation, and Disease. Immunity. 2019;50:1352–64.
Sun G, Guzman E, Balasanyan V, Conner C, Wong K, Zhou H, et al. A molecular signature for anastasis, recovery from the brink of apoptotic cell death. J Cell Biol. 2017;216:3355–68.
You W, Knoops K, Berendschot T, Benedikter B, Webers C, Reutelingsperger C, et al. PGC-1a mediated mitochondrial biogenesis promotes recovery and survival of neuronal cells from cellular degeneration. Cell Death Discov. 2024;10:180.
Zaitceva V, Kopeina G, Zhivotovsky B. Anastasis: Return Journey from Cell Death. Cancers. 2021;13:3671.
Tang HM, Fung MC, Tang HL Detecting Anastasis In Vivo by CaspaseTracker Biosensor. J Vis Exp. 2018;132:54107.
Mohammed R, Khosravi M, Rahman H, Adili A, Kamali N, Soloshenkov P, et al. Anastasis: cell recovery mechanisms and potential role in cancer. Cell Commun Signal. 2022;20:81.
Garrido C, Galluzzi L, Brunet M, Puig P, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–33.
Redza-Dutordoir M, Averill-Bates D. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863:2977–92.
Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9:459–70.
Tang H, Tang H, Hardwick J, Fung M Strategies for tracking anastasis, a cell survival phenomenon that reverses apoptosis. J Vis Exp. 2015;16:51964.
Karbowski M. Mitochondria on guard: role of mitochondrial fusion and fission in the regulation of apoptosis. Adv Exp Med Biol. 2010;687:131–42.
Wang S, Long H, Hou L, Feng B, Ma Z, Wu Y, et al. The mitophagy pathway and its implications in human diseases. Signal Transduct Target Ther. 2023;8:304.
Amin M, Abuo-Rahma G, Shaykoon M, Marzouk A, Abourehab M, Saraya R, et al. Design, synthesis, cytotoxic activities, and molecular docking of chalcone hybrids bearing 8-hydroxyquinoline moiety with dual tubulin/EGFR kinase inhibition. Bioorg Chem. 2023;134:106444.
Chinskey N, Zheng Q, Zacks D. Control of photoreceptor autophagy after retinal detachment: the switch from survival to death. Invest Ophthalmol Vis Sci. 2014;55:688–95.
Kaur B, Miglioranza Scavuzzi B, Yang M, Yao J, Jia L, Abcouwer S, et al. ER Stress and Mitochondrial Perturbations Regulate Cell Death in Retinal Detachment: Exploring the Role of HIF1α. Invest Ophthalmol Vis Sci. 2024;65:39.
Kaur B, Scavuzzi BM, Abcouwer SF, Zacks DN. A simplified protocol to induce hypoxia in a standard incubator: A focus on retinal cells. Exp Eye Res. 2023;236:1–22.
Scavuzzi BM, Shanmugam S, Yang M, Yao J, Hager H, Kaur B, et al. Remote Preconditioning Provides Protection Against Retinal Cell Death From Retinal Detachment. Invest Ophthalmol Visual Sci. 2025;66:34.
Mouse qPCR Primer Pair [Internet]. Available from: https://www.origene.com/catalog/gene-expression/qpcr-primer-pairs/mp212215/pum1-mouse-qpcr-primer-pair-nm_030722.
de Jonge H, Fehrmann R, de Bont E, Hofstra R, Gerbens F, Kamps W, et al. Evidence based selection of housekeeping genes. PLoS ONE. 2007;2:e898.
Feoktistova M, Geserick P, Leverkus M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb Protoc. 2016;2016:pdb.prot087379.
Dos Santos T, de Brito Sousa K, Andreo L, Martinelli A, Rodrigues M, Bussadori S, et al. Effect of Photobiomodulation on C2C12 Myoblasts Cultivated in M1 Macrophage-conditioned Media. Photochem Photobio. 2020;96:906–16.
Vandersickel V, Slabbert J, Thierens H, Vral A. Comparison of the colony formation and crystal violet cell proliferation assays to determine cellular radiosensitivity in a repair-deficient MCF10A cell line. Radiat Meas. 2011;46:72–5.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Yang M, Yao J, Jia L, Kocab A, Zacks D Preservation of retinal structure and function in two mouse models of inherited retinal degeneration by ONL1204, an inhibitor of the Fas receptor. Cell Death Dis. 2024;15:576.
Ross B, Jia L, Kong D, Wang T, Yao J, Hager H, et al. Hypoxia-Inducible Factor-1α in Rods Is Neuroprotective Following Retinal Detachment. Invest Ophthalmol Vis Sci. 2022;63:7.
Pan W, Wubben T, Besirli C Photoreceptor metabolic reprogramming: current understanding and therapeutic implications. Commun Biol. 2021;4:245.
Adebayo M, Singh S, Singh A, Dasgupta S Mitochondrial fusion and fission: The fine-tune balance for cellular homeostasis. FASEB J. 2021;35:e21620.
Xie L, Shi F, Li Y, Li W, Yu X, Zhao L, et al. Drp1-dependent remodeling of mitochondrial morphology triggered by EBV-LMP1 increases cisplatin resistance. Signal Transduct Target Ther. 2020;5:56.
Jiménez-Loygorri J, Benítez-Fernández R, Viedma-Poyatos Á, Zapata-Muñoz J, Villarejo-Zori B, Gómez-Sintes R, et al. Mitophagy in the retina: Viewing mitochondrial homeostasis through a new lens. Prog Retin Eye Res. 2023;96:101205.
She X, Lu X, Li T, Sun J, Liang J, Zhai Y, et al. Inhibition of Mitochondrial Fission Preserves Photoreceptors after Retinal Detachment. Am J Pathol. 2018;188:1713–22.
Yamazaki K, Ishida K, Otsu W, Muramatsu A, Nakamura S, Yamada W, et al. Delphinidins from Maqui Berry (Aristotelia chilensis) ameliorate the subcellular organelle damage induced by blue light exposure in murine photoreceptor-derived cells. BMC Complement Med Ther. 2024;24.
Yako T, Nakamura M, Nakamura S, Shimazawa M, Hara H Mitochondria fission by Drp1 as the potential therapeutic target for retinal pigmented epithelial cell death. Invest Ophthalmol Visual Sci. 2019;60:1948.
Wang F, Gao Y, Zhou L, Chen J, Xie Z, Ye Z, et al. USP30: Structure, Emerging Physiological Role, and Target Inhibition. Front Pharmacol. 2022;13:851654.
Li X, Wang T, Tao Y, Wang X, Li L, Liu J MF-094, a potent and selective USP30 inhibitor, accelerates diabetic wound healing by inhibiting the NLRP3 inflammasome. Exp Cell Res. 2022;410:112967.
Tang H, Yuen K, Tang H, Fung M. Reversibility of apoptosis in cancer cells. Br J Cancer. 2009;100:118–22.
Dhar K, Jena K, Mehto S, Sahu R, Murmu K, Mahajan A, et al. Programmed cell revival from imminent cell death enhances tissue repair and regeneration. EMBO J. 2025;44:5244–89.
Brzezinski J, Reh T. Photoreceptor cell fate specification in vertebrates. Development. 2015;142:3263–73.
Quintana-Cabrera R, Scorrano L. Determinants and outcomes of mitochondrial dynamics. Mol Cell. 2023;83:857–76.
Nilles J, Weiss J, Theile D. Crystal violet staining is a reliable alternative to bicinchoninic acid assay-based normalization. Biotechniques. 2022;73:131–5.
Sanvicens N, Gómez-Vicente V, Masip I, Messeguer A, Cotter T. Oxidative stress-induced apoptosis in retinal photoreceptor cells is mediated by calpains and caspases and blocked by the oxygen radical scavenger CR-6. J Biol Chem. 2004;279:39268–78.
Zhou B, Fang L, Dong Y, Yang J, Chen X, Zhang N, et al. Mitochondrial quality control protects photoreceptors against oxidative stress in the H2O2-induced models of retinal degeneration diseases. Cell Death Dis. 2021;12:413.
Kolesar J, Wang C, Taguchi Y, Chou S, Kaufman B Two-dimensional intact mitochondrial DNA agarose electrophoresis reveals the structural complexity of the mammalian mitochondrial genome. Nucleic Acids Res. 2013;41:e58.
Twig G, Elorza A, Molina AJA, Mohamed H, Wikstrom JD, Walzer G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–46.
Legros F, Lombès A, Frachon P, Rojo M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol Biol Cell. 2002;13:4343–54.
Ma K, Chen G, Li W, Kepp O, Zhu Y, Chen Q Mitophagy, Mitochondrial Homeostasis, and Cell Fate. Front Cell Dev Biol. 2020;8:467.
Mary Arnaud, Eysert Fanny, Checler F, Chami M. Mitophagy in Alzheimer’s disease: Molecular defects and therapeutic approaches. Mol Psychiatry. 2023;28:202–16.
Cen X, Chen Y, Xu X, Wu R, He F, Zhao Q, et al. Pharmacological targeting of MCL-1 promotes mitophagy and improves disease pathologies in an Alzheimer’s disease mouse model. Nature Commun. 2020;11:5731.
Lou G, Palikaras K, Lautrup S, Scheibye-Knudsen M, Tavernarakis N, Fang EF. Mitophagy and Neuroprotection. Trends Mol Med. 2020;26:8–20.
Doxaki C, Palikaras K. Neuronal Mitophagy: Friend or Foe?. Front Cell Dev Biol. 2021;8:611938.
Jeong D, Um J, Kim Y, Shin D, Im S, Lee K, et al. The Mst1/2-BNIP3 axis is required for mitophagy induction and neuronal viability under mitochondrial stress. Exp Mol Med. 2024;56:674–85.
Antico O, Thompson PW, Hertz NT, Muqit MMK, Parton LE. Targeting mitophagy in neurodegenerative diseases. Nature Rev Drug Discovery. 2025;24:276–299.
Schwarz GATL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31–42.
Aishwarya R, Alam S, Abdullah C, Morshed M, Nitu S, Panchatcharam M, et al. Pleiotropic effects of mdivi-1 in altering mitochondrial dynamics, respiration, and autophagy in cardiomyocytes. Redox Biol. 2020;36:101660.
Wykoff C, Flynn HWJ, Scott I. What is the optimal timing for rhegmatogenous retinal detachment repair?. JAMA Ophthalmol. 2013;131:1399–400.
Hassan T, Sarrafizadeh R, Ruby A, Garretson B, Kuczynski B, Williams G. The effect of duration of macular detachment on results after the scleral buckle repair of primary, macula-off retinal detachments. Ophthalmology. 2002;109:146–52.
Ross W, Kozy D. Visual recovery in macula-off rhegmatogenous retinal detachments. Ophthalmology. 1998;105:2149–53.
Cebulla C, Zelinka C, Scott M, Lubow M, Bingham A, Rasiah S, et al. A chick model of retinal detachment: cone rich and novel. PLoS ONE. 2012;7:e44257.
Augustine J, Pavlou S, Ali I, Harkin K, Ozaki E, Campbell M, et al. IL-33 deficiency causes persistent inflammation and severe neurodegeneration in retinal detachment. J Neuroinflammat. 2019;16:251.
Trichonas G, Murakami Y, Thanos A, Morizane Y, Kayama M, Debouck C, et al. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc Natl Acad Sci USA. 2010;107:21695–700.
Ranty M, Carpentier S, Cournot M, Rico-Lattes I, Malecaze F, Levade T, et al. Ceramide production associated with retinal apoptosis after retinal detachment. Graefes Arch Clin Exp Ophthalmol. 2009;247:215–24.
Acknowledgements
This work utilized the University of Michigan Vision Research Core, which is funded by P30 EY007003 from the National Eye Institute.
Funding
D.N.Z. was supported by NIH R01EY020823. D.N.Z. is also a 2025 Alcon Research Institute (“ARI”) Senior Investigator Grant Recipient, which supported this work. B.M.S. was supported by Training Grant T32AR07080 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Author information
Authors and Affiliations
Contributions
D.N.Z. study conceptualization, project supervision, and manuscript preparation. B.K., B.M.S. and J.Y. experimental design, experiments, data analysis and manuscript preparation. M.Y. and L.J. transient detachment experiments and data analysis. S.I.L. microscopy and image analysis. J.S. experiments and data analysis, A.K. experimental design and data analysis. S.S. flow cytometry experimental design, experiments, data analysis. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.N.Z. is an employee of the University of Michigan and ONL Therapeutics. D.N.Z. also holds patents through the University of Michigan that are licensed to ONL Therapeutics. A.K. is an employee of ONL Therapeutics. All other authors declare no conflict of interest.
Ethics statement
Animal experiments were performed following the protocols approved by the Institutional Animal Care & Use Committee (IACUC) at the University of Michigan and in compliance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Visual Research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Massimiliano Agostini
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaur, B., Miglioranza Scavuzzi, B., Yao, J. et al. Recovery from apoptosis in photoreceptor cells: A role for mitophagy. Cell Death Dis (2026). https://doi.org/10.1038/s41419-026-08436-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-026-08436-3