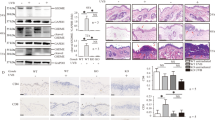
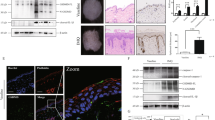
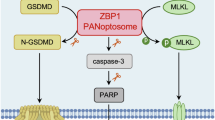
Abstract
Ultraviolet B (UVB) is a well-recognized trigger of cutaneous lupus erythematosus (CLE), yet its molecular basis remains largely undefined. Here, using single-cell transcriptomics and a lupus-prone mouse model, we identify keratinocyte-derived macrophage migration inhibitory factor (MIF) as a key amplifier of cutaneous inflammation through a self-sustaining feedback loop. Single-cell RNA sequencing reveals elevated MIF expression specifically within pathogenic, interferon-high keratinocyte subclusters associated with CLE, which is further validated across major CLE subtypes in clinical skin samples. In vitro, UVB irradiation dose-dependently induces the release of MIF from keratinocytes, which in turn promotes inflammatory signaling and matrix remodeling in both keratinocytes and fibroblasts. Mechanistically, we demonstrate that UVB irradiation activates the ribotoxic stress response (RSR), leading to the p38-C/EBPβ-mediated transcriptional upregulation of NLRP3 and GSDMD cleavage in keratinocytes. The ensuing GSDMD-dependent pyroptosis facilitates the release of MIF, primarily through GSDMD pores rather than vesicular secretion, which in turn amplifies the p38-C/EBPβ signaling pathway. Therapeutic disruption of this loop either by gene silencing via AAVs or pharmacological inhibition via microneedles, markedly attenuates epidermal hyperplasia and cytokine imbalance in lupus-prone mice. These findings uncover a previously unrecognized MIF-p38-GSDMD inflammatory loop contributes to the UVB-induced cutaneous lupus, offering both mechanistic insights and translational opportunities for CLE.

Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. This study involved the re-analysis of publicly available single-cell RNA sequencing data from GEO under accession number GSE186476. All of the data can be found in either the main text or the supplementary materials.
References
Kiriakidou M, Ching CL. Systemic lupus erythematosus. Ann Intern Med. 2020;172:ITC81–ITC96.
Wenzel J. Cutaneous lupus erythematosus: new insights into pathogenesis and therapeutic strategies. Nat Rev Rheumatol. 2019;15:519–32.
Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12:605–20.
Hart PH, Norval M, Byrne SN, Rhodes LE. Exposure to ultraviolet radiation in the modulation of human diseases. Annu Rev Pathol. 2019;14:55–81.
Curtiss P, Walker AM, Chong BF. A systematic review of the progression of cutaneous lupus to systemic lupus erythematosus. Front Immunol. 2022;13:866319.
Ertugrul G, Keles D, Oktay G, Aktan S. Matrix metalloproteinase-2 and -9 activity levels increase in cutaneous lupus erythematosus lesions and correlate with disease severity. Arch Dermatol Res. 2018;310:173–9.
Yu C, Chang C, Zhang J. Immunologic and genetic considerations of cutaneous lupus erythematosus: a comprehensive review. J Autoimmun. 2013;41:34–45.
Jiang Y, Tsoi LC, Billi AC, Ward NL, Harms PW, Zeng C, et al. Cytokinocytes: the diverse contribution of keratinocytes to immune responses in skin. JCI Insight. 2020;5:e142067.
Tian J, Shi L, Zhang D, Yao X, Zhao M, Kumari S, et al. Dysregulation in keratinocytes drives systemic lupus erythematosus onset. Cell Mol Immunol. 2025;22:83–96.
Billi AC, Ma F, Plazyo O, Gharaee-Kermani M, Wasikowski R, Hile GA, et al. Nonlesional lupus skin contributes to inflammatory education of myeloid cells and primes for cutaneous inflammation. Sci Transl Med. 2022;14:eabn2263.
Martinez BA, Shrotri S, Kingsmore KM, Bachali P, Grammer AC, Lipsky PE. Machine learning reveals distinct gene signature profiles in lesional and nonlesional regions of inflammatory skin diseases. Sci Adv. 2022;8:eabn4776.
Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151–9.
Kuhn A, Wenzel J, Weyd H. Photosensitivity, apoptosis, and cytokines in the pathogenesis of lupus erythematosus: a critical review. Clin Rev Allergy Immunol. 2014;47:148–62.
Klein B, Kunz M. Current concepts of photosensitivity in cutaneous lupus erythematosus. Front Med. 2022;9:939594.
Sanders CJ, Van Weelden H, Kazzaz GA, Sigurdsson V, Toonstra J, Bruijnzeel-Koomen CA. Photosensitivity in patients with lupus erythematosus: a clinical and photobiological study of 100 patients using a prolonged phototest protocol. Br J Dermatol. 2003;149:131–7.
Kuhn A, Wozniacka A, Szepietowski JC, Glaser R, Lehmann P, Haust M, et al. Photoprovocation in cutaneous lupus erythematosus: a multicenter study evaluating a standardized protocol. J Invest Dermatol. 2011;131:1622–30.
Wallace DJ, Figueras F, Wegener WA, Goldenberg DM. Experience with milatuzumab, an anti-CD74 antibody against immunomodulatory macrophage migration inhibitory factor (MIF) receptor, for systemic lupus erythematosus (SLE). Ann Rheum Dis. 2021;80:954–5.
Price S. Connective tissue diseases: small-molecule inhibitor of mIF protects lupus-prone mice from kidney disease. Nat Rev Rheumatol. 2011;7:70.
Sreih A, Ezzeddine R, Leng L, LaChance A, Yu G, Mizue Y, et al. Dual effect of the macrophage migration inhibitory factor gene on the development and severity of human systemic lupus erythematosus. Arthritis Rheum. 2011;63:3942–51.
Leng L, Chen L, Fan J, Greven D, Arjona A, Du X, et al. A small-molecule macrophage migration inhibitory factor antagonist protects against glomerulonephritis in lupus-prone NZB/NZW F1 and MRL/lpr mice. J Immunol. 2011;186:527–38.
Zhou Y, Chen H, Liu L, Yu X, Sukhova GK, Yang M, et al. CD74 Deficiency mitigates systemic lupus erythematosus-like autoimmunity and pathological findings in mice. J Immunol. 2017;198:2568–77.
De la Cruz-Mosso U, Bucala R, Palafox-Sanchez CA, Parra-Rojas I, Padilla-Gutierrez JR, Pereira-Suarez AL, et al. Macrophage migration inhibitory factor: association of -794 CATT5-8 and -173 G>C polymorphisms with TNF-alpha in systemic lupus erythematosus. Hum Immunol. 2014;75:33–9.
Lang T, Foote A, Lee JP, Morand EF, Harris J. MIF: implications in the pathoetiology of systemic lupus erythematosus. Front Immunol. 2015;6:577.
Shimizu T, Abe R, Ohkawara A, Nishihira J. Ultraviolet B radiation upregulates the production of macrophage migration inhibitory factor (MIF) in human epidermal keratinocytes. J Invest Dermatol. 1999;112:210–5.
Heise R, Vetter-Kauczok CS, Skazik C, Czaja K, Marquardt Y, Lue H, et al. Expression and function of macrophage migration inhibitory factor in the pathogenesis of UV-induced cutaneous nonmelanoma skin cancer. Photochem Photobiol. 2012;88:1157–64.
Yoshihisa Y, Rehman MU, Shimizu T. Astaxanthin, a xanthophyll carotenoid, inhibits ultraviolet-induced apoptosis in keratinocytes. Exp Dermatol. 2014;23:178–83.
Yoshihisa Y, Andoh T, Rehman MU, Shimizu T. The regulation of protein kinase casein kinase II by apigenin is involved in the inhibition of ultraviolet B-induced macrophage migration inhibitory factor-mediated hyperpigmentation. Phytother Res. 2020;34:1320–8.
Yoshihisa Y, Norisugi O, Matsunaga K, Nishihira J, Shimizu T. Involvement of MIF in basement membrane damage in chronically UVB-exposed skin in mice. PLoS ONE. 2014;9:e89569.
Watanabe H, Shimizu T, Nishihira J, Abe R, Nakayama T, Taniguchi M, et al. Ultraviolet A-induced production of matrix metalloproteinase-1 is mediated by macrophage migration inhibitory factor (MIF) in human dermal fibroblasts. J Biol Chem. 2004;279:1676–83.
Klein B, Reynolds MB, Xu B, Gharaee-Kermani M, Gao Y, Berthier CC, et al. Epidermal ZBP1 stabilizes mitochondrial Z-DNA to drive UV-induced IFN signaling in autoimmune photosensitivity. Sci Immunol. 2025;10:eado1710.
Gomez-Banuelos E, Goldman DW, Andrade V, Darrah E, Petri M, Andrade F. Uncoupling interferons and the interferon signature explains clinical and transcriptional subsets in SLE. Cell Rep Med. 2024;5:101569.
Carter LM, Wigston Z, Laws P, Vital EM. Rapid efficacy of anifrolumab across multiple subtypes of recalcitrant cutaneous lupus erythematosus parallels changes in discrete subsets of blood transcriptomic and cellular biomarkers. Br J Dermatol. 2023;189:210–8.
Lang T, Lee JPW, Elgass K, Pinar AA, Tate MD, Aitken EH, et al. Macrophage migration inhibitory factor is required for NLRP3 inflammasome activation. Nat Commun. 2018;9:2223.
Shin MS, Kang Y, Wahl ER, Park HJ, Lazova R, Leng L, et al. Macrophage migration inhibitory factor regulates U1 small nuclear RNP immune complex-mediated activation of the NLRP3 inflammasome. Arthritis Rheumatol. 2019;71:109–20.
Al-Abed Y, VanPatten S. MIF as a disease target: ISO-1 as a proof-of-concept therapeutic. Future Med Chem. 2011;3:45–63.
Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800.
Robinson KS, Toh GA, Rozario P, Chua R, Bauernfried S, Sun Z, et al. ZAKalpha-driven ribotoxic stress response activates the human NLRP1 inflammasome. Science. 2022;377:328–35.
Zhou JY, Sarkar MK, Okamura K, Harris JE, Gudjonsson JE, Fitzgerald KA. Activation of the NLRP1 inflammasome in human keratinocytes by the dsDNA mimetic poly(dA:dT). Proc Natl Acad Sci USA. 2023;120:e2213777120.
Jenster LM, Lange KE, Normann S, vom Hemdt A, Wuerth JD, Schiffelers LDJ, et al. P38 kinases mediate NLRP1 inflammasome activation after ribotoxic stress response and virus infection. J Exp Med. 2023;220:e20220837.
Scholtissek B, Zahn S, Maier J, Klaeschen S, Braegelmann C, Hoelzel M, et al. Immunostimulatory endogenous nucleic acids drive the lesional inflammation in cutaneous lupus erythematosus. J Invest Dermatol. 2017;137:1484–92.
Gehrke N, Mertens C, Zillinger T, Wenzel J, Bald T, Zahn S, et al. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity. 2013;39:482–95.
Zhou B, Jiang ZH, Dai MR, Ai YL, Xiao L, Zhong CQ, et al. Full-length GSDME mediates pyroptosis independent from cleavage. Nat Cell Biol. 2024;26:1545–57.
Zhong FL, Mamai O, Sborgi L, Boussofara L, Hopkins R, Robinson K, et al. Germline NLRP1 mutations cause skin inflammatory and cancer susceptibility syndromes via inflammasome activation. Cell. 2016;167:187–202.e17.
Fenini G, Grossi S, Contassot E, Biedermann T, Reichmann E, French LE, et al. Genome editing of human primary keratinocytes by CRISPR/Cas9 reveals an essential role of the NLRP1 inflammasome in UVB sensing. J Invest Dermatol. 2018;138:2644–52.
Tang N, Liu XT, Wen WL, Liang TS, Lv XT, Li QL, et al. Restraint stress promotes monobenzone-induced depigmentation in mice via the activation of glucocorticoid receptor/macrophage migration inhibitory factor signaling pathway. Mol Immunol. 2023;161:33–43.
Hernandez-Pigeon H, Jean C, Charruyer A, Haure MJ, Baudouin C, Charveron M, et al. UVA induces granzyme B in human keratinocytes through MIF: implication in extracellular matrix remodeling. J Biol Chem. 2007;282:8157–64.
Gesser B, Rasmussen MK, Raaby L, Rosada C, Johansen C, Kjellerup RB, et al. Dimethylfumarate inhibits MIF-induced proliferation of keratinocytes by inhibiting MSK1 and RSK1 activation and by inducing nuclear p-c-Jun (S63) and p-p53 (S15) expression. Inflamm Res. 2011;60:643–53.
Engelman JA, Lisanti MP, Scherer PE. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis. J Biol Chem. 1998;273:32111–20.
Luo L, Pasquali L, Srivastava A, Freisenhausen JC, Pivarcsi A, Sonkoly E. The long noncoding RNA LINC00958 is induced in psoriasis epidermis and modulates epidermal proliferation. J Invest Dermatol. 2023;143:999–1010.
Li JP, Qiu S, Tai GJ, Liu YM, Wei W, Fu MM, et al. NLRP3 inflammasome-modulated angiogenic function of EPC via PI3K/ Akt/mTOR pathway in diabetic myocardial infarction. Cardiovasc Diabetol. 2025;24:6.
Vind AC, Wu Z, Firdaus MJ, Snieckute G, Toh GA, Jessen M, et al. The ribotoxic stress response drives acute inflammation, cell death, and epidermal thickening in UV-irradiated skin in vivo. Mol Cell. 2024;84:4774–4789.e9.
Cummins DL, Gaspari AA. Photoprotection by thalidomide in patients with chronic cutaneous and systemic lupus erythematosus: discordant effects on minimal erythema dose and sunburn cell formation. Br J Dermatol. 2004;151:458–64.
Sarkar MK, Hile GA, Tsoi LC, Xing X, Liu J, Liang Y, et al. Photosensitivity and type I IFN responses in cutaneous lupus are driven by epidermal-derived interferon kappa. Ann Rheum Dis. 2018;77:1653–64.
Stannard JN, Reed TJ, Myers E, Lowe L, Sarkar MK, Xing X, et al. Lupus skin is primed for IL-6 inflammatory responses through a keratinocyte-mediated autocrine type I interferon loop. J Invest Dermatol. 2017;137:115–22.
Bernard JJ, Cowing-Zitron C, Nakatsuji T, Muehleisen B, Muto J, Borkowski AW, et al. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat Med. 2012;18:1286–90.
Psarras A, Vital EM. Keratinocytes: from passive targets to active mediators of systemic autoimmunity. Sci Transl Med. 2022;14:eabo3961.
Werth VP, Furie RA, Romero-Diaz J, Navarra S, Kalunian K, van Vollenhoven RF, et al. Trial of Anti-BDCA2 antibody litifilimab for cutaneous lupus erythematosus. N Engl J Med. 2022;387:321–31.
Vital EM, Wittmann M, Edward S, Md Yusof MY, MacIver H, Pease CT, et al. Brief report: responses to rituximab suggest B cell-independent inflammation in cutaneous systemic lupus erythematosus. Arthritis Rheumatol. 2015;67:1586–91.
Noh SU, Park YM. The effect of green tea polyphenols on macrophage migration inhibitory factor-associated steroid resistance. Br J Dermatol. 2012;166:653–7.
Li T, Sun H, Li Y, Su L, Jiang J, Liu Y, et al. Downregulation of macrophage migration inhibitory factor attenuates NLRP3 inflammasome mediated pyroptosis in sepsis-induced AKI. Cell Death Discov. 2022;8:61.
Chen W, Ge L, Zhang C. The molecular mechanism of berberine affecting psoriasis skin inflammation by regulating keratinocyte pyroptosis via the p38 MAPK/NF-kappaB pathway. Naunyn Schmiedebergs Arch Pharmacol. 2025;398:3843–59.
Yu WJ, Jiang WX, Liu SJ, Li HH, Lin QY. Single-cell RNA sequencing reveals that myeloid S100A8/A9 is a novel regulator of the transition from adaptive hypertrophy to heart failure after pressure overload. Theranostics. 2025;15:8587–608.
Chen L, Gong P, Su Y, Meng L, Wang M, Gao W, et al. Angiotensin II type 2 receptor agonist attenuates LPS-induced acute lung injury through modulating THP-1-derived macrophage reprogramming. Naunyn Schmiedebergs Arch Pharmacol. 2024;397:99–108.
Niebel D, de Vos L, Fetter T, Bragelmann C, Wenzel J. Cutaneous lupus erythematosus: an update on pathogenesis and future therapeutic directions. Am J Clin Dermatol. 2023;24:521–40.
Salle R, Chasset F, Kottler D, Picard-Dahan C, Jannic A, Mekki N, et al. Belimumab for refractory manifestations of cutaneous lupus: a multicenter, retrospective observational study of 16 patients. J Am Acad Dermatol. 2020;83:1816–9.
Manzi S, Sanchez-Guerrero J, Merrill JT, Furie R, Gladman D, Navarra SV, et al. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Ann Rheum Dis. 2012;71:1833–8.
Chasset F, Jaume L, Mathian A, Abisror N, Dutheil A, Barbaud A, et al. Rapid efficacy of anifrolumab in refractory cutaneous lupus erythematosus. J Am Acad Dermatol. 2023;89:171–3.
Han X, Vesely MD, Yang W, Sanmamed MF, Badri T, Alawa J, et al. PD-1H (VISTA)-mediated suppression of autoimmunity in systemic and cutaneous lupus erythematosus. Sci Transl Med. 2019;11:eaax1159.
Yang JQ, Chun T, Liu H, Hong S, Bui H, Van Kaer L, et al. CD1d deficiency exacerbates inflammatory dermatitis in MRL-lpr/lpr mice. Eur J Immunol. 2004;34:1723–32.
Kuhn A, Sticherling M, Bonsmann G. Clinical manifestations of cutaneous lupus erythematosus. J Dtsch Dermatol Ges. 2007;5:1124–37.
Guo C, Liang L, Zheng J, Xie Y, Qiu X, Tan G, et al. UCHL1 aggravates skin fibrosis through an IGF-1-induced Akt/mTOR/HIF-1alpha pathway in keloid. FASEB J. 2023;37:e23015.
Park HJ, Kim HJ, Lee JH, Lee JY, Cho BK, Kang JS, et al. Corticotropin-releasing hormone (CRH) downregulates interleukin-18 expression in human HaCaT keratinocytes by activation of p38 mitogen-activated protein kinase (MAPK) pathway. J Invest Dermatol. 2005;124:751–5.
Park HJ, Kim HJ, Lee JY, Cho BK, Gallo RL, Cho DH. Adrenocorticotropin hormone stimulates interleukin-18 expression in human HaCaT keratinocytes. J Invest Dermatol. 2007;127:1210–6.
Lian N, Chen Y, Chen S, Zhang Y, Chen H, Yang Y, et al. Gasdermin D-mediated keratinocyte pyroptosis as a key step in psoriasis pathogenesis. Cell Death Dis. 2023;14:595.
Yin H, Chen CY, Liu YW, Tan YJ, Deng ZL, Yang F, et al. Synechococcus elongatus PCC7942 secretes extracellular vesicles to accelerate cutaneous wound healing by promoting angiogenesis. Theranostics. 2019;9:2678–93.
Broome AM, Eckert RL. Microtubule-dependent redistribution of a cytoplasmic cornified envelope precursor. J Invest Dermatol. 2004;122:29–38.
Wang CJ, Zhou ZG, Holmqvist A, Zhang H, Li Y, Adell G, et al. Survivin expression quantified by Image Pro-Plus compared with visual assessment. Appl Immunohistochem Mol Morphol. 2009;17:530–5.
Acknowledgements
We are deeply grateful to Professor Tao Liu and his research group for their expert contribution and collaboration in the engineering of the dissolvable microneedle patches. Furthermore, we sincerely acknowledge the dedicated staff at the Basic and Translational Medical Research Center of Sun Yat-sen Memorial Hospital, Sun Yat-sen University, for providing critical platform resources and technical support throughout this project.
Funding
This work was supported by the following grants: National Natural Science Foundation of China Grant No. 81970632 (YZ, Guangzhou), National Natural Science Foundation of China Grant No. 81872524, 82073431 (LW, Guangzhou), Guangdong Science and Technology Department Grant No. 2020B1212060018 and 2020B1212030004 (YZ, Guangzhou), Sun Yat-sen Pilot Scientific Research Fund Grant No. YXQH202415 (CG, Guangzhou).
Author information
Authors and Affiliations
Contributions
YZ, CG, and TL designed the experiment. CG, LW, and YZ funded the experiment. CG, SL, XY, JC, YD, HL, HL, and LW performed the in vitro and in vivo experiments, analyzed and generated data. SL performed the bioinformatics analysis of scRNA-seq data. TL and JL fabricated the microneedle patches. All authors participated writing and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Youwei Ai
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, C., Luo, S., Luo, J. et al. A MIF-p38-GSDMD inflammatory loop in keratinocytes underlies UVB-induced cutaneous lupus. Cell Death Dis (2026). https://doi.org/10.1038/s41419-026-08443-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-026-08443-4