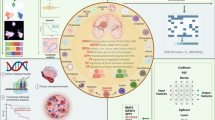
Abstract
Glioma, the most prevalent primary brain tumor, primarily arises from glial cells or their progenitors. Histologically, gliomas are classified into astrocytomas, oligodendrogliomas, and ependymomas. Due to their aggressive invasive nature and resistance to chemotherapy, gliomas exhibit high recurrence rates and poor clinical outcomes. Regulated cell death (RCD) refers to a set of genetically controlled cellular processes that significantly influence tumor behavior. RCD plays a dual role in cancer: under normal physiological conditions, it eliminates malignant cells to prevent tumorigenesis, while in pathological conditions, tumor cells evade RCD to gain survival advantages. Furthermore, distinct RCD pathways can modulate the tumor immune microenvironment, thereby affecting therapeutic outcomes. Targeting RCD mechanisms presents a promising strategy to overcome therapeutic resistance and advance innovative glioma immunotherapies. This review explores the molecular mechanisms of pyroptosis, ferroptosis, necroptosis, and autophagy in glioma, emphasizing their critical roles in tumor progression. It also examines therapeutic strategies targeting RCD, including recent advancements in glutathione peroxidase 4 (GPX4) inhibitors, oncolytic virotherapy, and other emerging agents. Furthermore, the review discusses the potential of nanoparticle-based drug delivery systems and multi-omics approaches to optimize personalized combination therapies, aiming to enhance multimodal, synergistic interventions for more effective glioma management.
Similar content being viewed by others
References
Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013-7. Neuro Oncol. 2020;22:iv1–iv96.
Smith HL, Wadhwani N, Horbinski C. Major Features of the 2021 WHO Classification of CNS Tumors. Neurotherapeutics. 2022;19:1691–704.
van den Bent MJ, Geurts M, French PJ, Smits M, Capper D, Bromberg JEC, et al. Primary brain tumours in adults. Lancet. 2023;402:1564–79.
Kim J, Zhu Y, Chen S, Wang D, Zhang S, Xia J, et al. Anti-glioma effect of ginseng-derived exosomes-like nanoparticles by active blood-brain-barrier penetration and tumor microenvironment modulation. J Nanobiotechnology. 2023;21:253.
Tong X, Tang R, Xiao M, Xu J, Wang W, Zhang B, et al. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol. 2022;15:174.
Cerella C, Teiten MH, Radogna F, Dicato M, Diederich M. From nature to bedside: pro-survival and cell death mechanisms as therapeutic targets in cancer treatment. Biotechnol Adv. 2014;32:1111–22.
Gao J, Xiong A, Liu J, Li X, Wang J, Zhang L, et al. PANoptosis: bridging apoptosis, pyroptosis, and necroptosis in cancer progression and treatment. Cancer Gene Ther. 2024;31:970–83.
Kaczor S, Szewczyk-Roszczenko O, Pawlak D, Hermanowicz A, Hermanowicz JM. GSDM family and glioma. Biochim Biophys Acta Rev Cancer. 2025;1880:189283.
Peng F, Liao M, Qin R, Zhu S, Peng C, Fu L, et al. Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduct Target Ther. 2022;7:286.
Guo D, Liu Z, Zhou J, Ke C, Li D. Significance of Programmed Cell Death Pathways in Neurodegenerative Diseases. Int J Mol Sci. 2024;25:9947.
Zheng J, Zhou Z, Qiu Y, Wang M, Yu H, Wu Z, et al. A Pyroptosis-Related Gene Prognostic Index Correlated with Survival and Immune Microenvironment in Glioma. J Inflamm Res. 2022;15:17–32.
Fu J, Li D, Zhang L, Maghsoudloo M, Cheng J, Fu J. Comprehensive analysis, diagnosis, prognosis, and cordycepin (CD) regulations for GSDME expressions in pan-cancers. Cancer Cell Int. 2024;24:279.
Li D, Zhang Z, Wang L. Emerging role of tumor microenvironmental nutrients and metabolic molecules in ferroptosis: Mechanisms and clinical implications. Biomed Pharmacother. 2024;179:117406.
Cirotti C, Taddei I, Contadini C, Di Girolamo C, Pepe G, De Bardi M, et al. NRF2 connects Src tyrosine kinase to ferroptosis resistance in glioblastoma. Life Sci Alliance. 2024;7:e202302205.
Yapici FI, Bebber CM, von Karstedt S. A guide to ferroptosis in cancer. Mol Oncol. 2024;18:1378–96.
Sanati M, Amin Yavari S. Liposome-integrated hydrogel hybrids: Promising platforms for cancer therapy and tissue regeneration. J Control Release. 2024;368:703–27.
Du R, Tripathi S, Najem H, Brat DJ, Lukas RV, Zhang P, et al. Glioblastoma Phagocytic Cell Death: Balancing the Opportunities for Therapeutic Manipulation. Cells. 2024;13:823.
Cui J, Shen HM, Lim LHK. The Role of Autophagy in Liver Cancer: Crosstalk in Signaling Pathways and Potential Therapeutic Targets. Pharmaceuticals (Basel). 2020;13:432.
Mokarram P, Albokashy M, Zarghooni M, Moosavi MA, Sepehri Z, Chen QM, et al. New frontiers in the treatment of colorectal cancer: Autophagy and the unfolded protein response as promising targets. Autophagy. 2017;13:781–819.
Guo Z, Guozhang H, Wang H, Li Z, Liu N. Ampelopsin inhibits human glioma through inducing apoptosis and autophagy dependent on ROS generation and JNK pathway. Biomed Pharmacother. 2019;116:108524.
Xiaojie Z, Bufu T, Jinhua L, Yang Y, Qiaoyou W, Shiji F, et al. Cuproptosis, ferroptosis and PANoptosis in tumor immune microenvironment remodeling and immunotherapy: culprits or new hope. Mol Cancer. 2024;23:255.
Samir P, Malireddi RKS, Kanneganti TD. The PANoptosome: A Deadly Protein Complex Driving Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front Cell Infect Microbiol. 2020;10:238.
Karmakar M, Minns M, Greenberg EN, Diaz-Aponte J, Pestonjamasp K, Johnson JL, et al. N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nat Commun. 2020;11:2212.
Hu Y, Liu Y, Zong L, Zhang W, Liu R, Xing Q, et al. The multifaceted roles of GSDME-mediated pyroptosis in cancer: therapeutic strategies and persisting obstacles. Cell Death Dis. 2023;14:836.
Frank D, Vince JE. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 2019;26:99–114.
Lee S, Karki R, Wang Y, Nguyen LN, Kalathur RC, Kanneganti TD. AIM2 forms a complex with pyrin and ZBP1 to drive PANoptosis and host defence. Nature. 2021;597:415–9.
Zhang R, Song Q, Lin X, Du B, Geng D, Gao D. GSDMA at the crossroads between pyroptosis and tumor immune evasion in glioma. Biochem Biophys Res Commun. 2023;686:149181.
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–w102.
Zeng Y, Cai Y, Chai P, Mao Y, Chen Y, Wang L, et al. Optimization of cancer immunotherapy through pyroptosis: A pyroptosis-related signature predicts survival benefit and potential synergy for immunotherapy in glioma. Front Immunol. 2022;13:961933.
Gao Z, Yang J. GNB4 Silencing Promotes Pyroptosis to Inhibit the Development of Glioma by Activating cGAS-STING Pathway. Mol Biotechnol. 2025;67:2262–76.
Cao Q, Wang X, Liu J, Dong Y, Wu X, Mi Y, et al. ICBP90, an epigenetic regulator, induces DKK3 promoter methylation, promotes glioma progression, and reduces sensitivity to cis-platinum. Exp Cell Res. 2024;436:113976.
Abu-Serie MM, Osuka S, Heikal LA, Teleb M, Barakat A, Dudeja V. Diethyldithiocarbamate-ferrous oxide nanoparticles inhibit human and mouse glioblastoma stemness: aldehyde dehydrogenase 1A1 suppression and ferroptosis induction. Front Pharmacol. 2024;15:1363511.
Gao W, Wang X, Zhou Y, Wang X, Yu Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct Target Ther. 2022;7:196.
Tu S, Zou Y, Yang M, Zhou X, Zheng X, Jiang Y, et al. Ferroptosis in hepatocellular carcinoma: Mechanisms and therapeutic implications. Biomed Pharmacother. 2025;182:117769.
Li S, He Y, Chen K, Sun J, Zhang L, He Y, et al. RSL3 Drives Ferroptosis through NF-κB Pathway Activation and GPX4 Depletion in Glioblastoma. Oxid Med Cell Longev. 2021;2021:2915019.
Sun S, Guo C, Gao T, Ma D, Su X, Pang Q, et al. Hypoxia Enhances Glioma Resistance to Sulfasalazine-Induced Ferroptosis by Upregulating SLC7A11 via PI3K/AKT/HIF-1α Axis. Oxid Med Cell Longev. 2022;2022:7862430.
Liu X, Cao Z, Wang W, Zou C, Wang Y, Pan L, et al. Engineered Extracellular Vesicle-Delivered CRISPR/Cas9 for Radiotherapy Sensitization of Glioblastoma. ACS Nano. 2023;17:16432–47.
Miao Z, Xu L, Gu W, Ren Y, Li R, Zhang S, et al. A targetable PRR11-DHODH axis drives ferroptosis- and temozolomide-resistance in glioblastoma. Redox Biol. 2024;73:103220.
Wang TX, Liang JY, Zhang C, Xiong Y, Guan KL, Yuan HX. The oncometabolite 2-hydroxyglutarate produced by mutant IDH1 sensitizes cells to ferroptosis. Cell Death Dis. 2019;10:755.
Tang X, Fu X, Liu Y, Yu D, Cai SJ, Yang C. Blockade of Glutathione Metabolism in IDH1-Mutated Glioma. Mol Cancer Ther. 2020;19:221–30.
Tsuchihashi K, Okazaki S, Ohmura M, Ishikawa M, Sampetrean O, Onishi N. et al. The EGF Receptor Promotes the Malignant Potential of Glioma by Regulating Amino Acid Transport System xc(. Cancer Res. 2016;76:2954–63.
Liu Y, Chou FJ, Lang F, Zhang M, Song H, Zhang W, et al. Protein Kinase B (PKB/AKT) Protects IDH-Mutated Glioma from Ferroptosis via Nrf2. Clin Cancer Res. 2023;29:1305–16.
Zheng XJ, Chen WL, Yi J, Li W, Liu JY, Fu WQ, et al. Apolipoprotein C1 promotes glioblastoma tumorigenesis by reducing KEAP1/NRF2 and CBS-regulated ferroptosis. Acta Pharmacol Sin. 2022;43:2977–92.
Liu T, Zhu C, Chen X, Guan G, Zou C, Shen S, et al. Ferroptosis, as the most enriched programmed cell death process in glioma, induces immunosuppression and immunotherapy resistance. Neuro Oncol. 2022;24:1113–25.
Meng X, Wang Z, Yang Q, Liu Y, Gao Y, Chen H, et al. Intracellular C5aR1 inhibits ferroptosis in glioblastoma through METTL3-dependent m6A methylation of GPX4. Cell Death Dis. 2024;15:729.
Yee PP, Wei Y, Kim SY, Lu T, Chih SY, Lawson C, et al. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat Commun. 2020;11:5424.
Lu T, Yee PP, Chih SY, Tang M, Chen H, Aregawi DG, et al. LC3-associated phagocytosis of neutrophils triggers tumor ferroptotic cell death in glioblastoma. Embo j. 2024;43:2582–605.
Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–16.
Chen D, Yu J, Zhang L. Necroptosis: an alternative cell death program defending against cancer. Biochim Biophys Acta. 2016;1865:228–36.
Rius-Pérez S, Pérez S, Toledano MB, Sastre J. Mitochondrial Reactive Oxygen Species and Lytic Programmed Cell Death in Acute Inflammation. Antioxid Redox Signal. 2023;39:708–27.
Zhou Z, Xu J, Huang N, Tang J, Ma P, Cheng Y. Clinical and Biological Significance of a Necroptosis-Related Gene Signature in Glioma. Front Oncol. 2022;12:855434.
Park S, Hatanpaa KJ, Xie Y, Mickey BE, Madden CJ, Raisanen JM, et al. The receptor interacting protein 1 inhibits p53 induction through NF-kappaB activation and confers a worse prognosis in glioblastoma. Cancer Res. 2009;69:2809–16.
Wang HL, Chang JC, Fang LW, Hsu HF, Lee LC, Yang JF, et al. Bulnesia sarmientoi Supercritical Fluid Extract Exhibits Necroptotic Effects and Anti-Metastatic Activity on Lung Cancer Cells. Molecules. 2018;23:3304.
Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D’Cruz AA, et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun. 2015;6:6282.
Goodall ML, Fitzwalter BE, Zahedi S, Wu M, Rodriguez D, Mulcahy-Levy JM, et al. The Autophagy Machinery Controls Cell Death Switching between Apoptosis and Necroptosis. Dev Cell. 2016;37:337–49.
Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–6.
Lv D, Han X, Hao L, Sun Z, Zhang A, Liu J, et al. Cysteine‑ and glycine‑rich protein 2: A vital regulator that inhibits necroptosis glioma cell by activating the JAK‑STAT1 pathways. Oncol Rep. 2025;53:40.
Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res. 2014;24:24–41.
Liu CC, Lin YC, Chen YH, Chen CM, Pang LY, Chen HA, et al. Cul3-KLHL20 Ubiquitin Ligase Governs the Turnover of ULK1 and VPS34 Complexes to Control Autophagy Termination. Mol Cell. 2016;61:84–97.
Li Y, Xu M, Ding X, Yan C, Song Z, Chen L, et al. Protein kinase C controls lysosome biogenesis independently of mTORC1. Nat Cell Biol. 2016;18:1065–77.
Cheng X, Ma X, Ding X, Li L, Jiang X, Shen Z, et al. Pacer Mediates the Function of Class III PI3K and HOPS Complexes in Autophagosome Maturation by Engaging Stx17. Mol Cell. 2017;65:1029–43.e5.
Zheng W, Chen Q, Liu H, Zeng L, Zhou Y, Liu X, et al. SDC1-dependent TGM2 determines radiosensitivity in glioblastoma by coordinating EPG5-mediated fusion of autophagosomes with lysosomes. Autophagy. 2023;19:839–57.
Xing Y, Wei X, Liu Y, Wang MM, Sui Z, Wang X, et al. Autophagy inhibition mediated by MCOLN1/TRPML1 suppresses cancer metastasis via regulating a ROS-driven TP53/p53 pathway. Autophagy. 2022;18:1932–54.
Geng F, Zhong Y, Su H, Lefai E, Magaki S, Cloughesy TF, et al. SREBP-1 upregulates lipophagy to maintain cholesterol homeostasis in brain tumor cells. Cell Rep. 2023;42:112790.
Huang T, Xu T, Wang Y, Zhou Y, Yu D, Wang Z, et al. Cannabidiol inhibits human glioma by induction of lethal mitophagy through activating TRPV4. Autophagy. 2021;17:3592–606.
Meyer N, Henkel L, Linder B, Zielke S, Tascher G, Trautmann S, et al. Autophagy activation, lipotoxicity and lysosomal membrane permeabilization synergize to promote pimozide- and loperamide-induced glioma cell death. Autophagy. 2021;17:3424–43.
Newton K, Strasser A, Kayagaki N, Dixit VM. Cell death. Cell. 2024;187:235–56.
Chen Z, Luo Y, Jia Q, Yang Z, Liu Z, Cui C, et al. NIR-II Fluorescence Imaging-guided Photothermal Activated Pyroptosis For Precision Therapy Of Glioma. Chembiochem. 2025;26:e202400804.
Zhang Y, Xi K, Fu Z, Zhang Y, Cheng B, Feng F, et al. Stimulation of tumoricidal immunity via bacteriotherapy inhibits glioblastoma relapse. Nat Commun. 2024;15:4241.
Song M, Tian J, Wang L, Dong S, Fu K, Chen S, et al. Efficient Delivery of Lomitapide using Hybrid Membrane-Coated Tetrahedral DNA Nanostructures for Glioblastoma Therapy. Adv Mater. 2024;36:e2311760.
Lu Y, He W, Huang X, He Y, Gou X, Liu X, et al. Strategies to package recombinant Adeno-Associated Virus expressing the N-terminal gasdermin domain for tumor treatment. Nat Commun. 2021;12:7155.
Gao X, Tang X, Tu Z, Yu J, Bao Y, Long G, et al. Tertiary amine modification enables triterpene nanoparticles to target the mitochondria and treat glioblastoma via pyroptosis induction. Biomaterials. 2025;317:123035.
Ye Y, Ren K, Dong Y, Yang L, Zhang D, Yuan Z, et al. Mitochondria-Targeting Pyroptosis Amplifier of Lonidamine-Modified Black Phosphorus Nanosheets for Glioblastoma Treatments. ACS Appl Mater Interfaces. 2023;15:26285–97.
Li S, Zeng F, Zhou Q, Li L, Lo H, Chen J, et al. NIR-II Photoacoustic Imaging-Guided Chemo-Photothermal Therapy Using PA1094T Combined with Anti-CD47 Antibody: Activating Pyroptosis against Orthotopic Glioblastoma. Adv Healthc Mater. 2025;14:e2403108.
Zarska M, Novak O, Jakubcova T, Novotny F, Urbancokova A, Havel F, et al. Photothermal induction of pyroptosis in malignant glioma spheroids using (16-mercaptohexadecyl)trimethylammonium bromide-modified cationic gold nanorods. Colloids Surf B Biointerfaces. 2024;243:114128.
Qu S, Qi S, Zhang H, Li Z, Wang K, Zhu T, et al. Albumin-bound paclitaxel augment temozolomide treatment sensitivity of glioblastoma cells by disrupting DNA damage repair and promoting ferroptosis. J Exp Clin Cancer Res. 2023;42:285.
Zhou H, Wang YX, Wu M, Lan X, Xiang D, Cai R, et al. FANCD2 deficiency sensitizes SHH medulloblastoma to radiotherapy via ferroptosis. J Pathol. 2024;262:427–40.
Deng L, Di Y, Chen C, Xia J, Lei B, Li N, et al. Depletion of the N(6)-Methyladenosine (m6A) reader protein IGF2BP3 induces ferroptosis in glioma by modulating the expression of GPX4. Cell Death Dis. 2024;15:181.
Wu H, Cao P, Wang H, Wang W, Yu H, You C, et al. Postoperative Injection of a Triptolide-Preloaded Hydrogel Prevents the Recurrence of Glioblastoma by Dual-Pathway Activation of Ferroptosis. Small. 2024;20:e2406036.
Zhao LX, Gong ZQ, Zhang Q, He DL, Ge RL, Meng J, et al. Graphdiyne nanoplatforms for photothermal-ferroptosis combination therapy against glioblastoma. J Control Release. 2023;359:12–25.
Fan R, Chen C, Mu M, Chuan D, Liu H, Hou H, et al. Engineering MMP-2 Activated Nanoparticles Carrying B7-H3 Bispecific Antibodies for Ferroptosis-Enhanced Glioblastoma Immunotherapy. ACS Nano. 2023;17:9126–39.
Neitzel LR, Fuller DT, Williams CH, Hong CC. Inhibition of GPR68 kills glioblastoma in zebrafish xenograft models. BMC Res Notes. 2024;17:235.
Shao G, Cui X, Wang Y, Luo S, Li C, Jiang Y, et al. Targeting MS4A4A: A novel pathway to improve immunotherapy responses in glioblastoma. CNS Neurosci Ther. 2024;30:e14791.
Lu S, Wang XZ, He C, Wang L, Liang SP, Wang CC, et al. ATF3 contributes to brucine-triggered glioma cell ferroptosis via promotion of hydrogen peroxide and iron. Acta Pharmacol Sin. 2021;42:1690–702.
Mansuer M, Zhou L, Wang C, Gao L, Jiang Y. Erianin induces ferroptosis in GSCs via REST/LRSAM1 mediated SLC40A1 ubiquitination to overcome TMZ resistance. Cell Death Dis. 2024;15:522.
Zhang G, Hu J, Li A, Zhang H, Guo Z, Li X, et al. Ginsenoside Rg5 inhibits glioblastoma by activating ferroptosis via NR3C1/HSPB1/NCOA4. Phytomedicine. 2024;129:155631.
Upadhyayula PS, Higgins DM, Mela A, Banu M, Dovas A, Zandkarimi F, et al. Dietary restriction of cysteine and methionine sensitizes gliomas to ferroptosis and induces alterations in energetic metabolism. Nat Commun. 2023;14:1187.
Zhou Z, Lu B, Wang C, Wang Z, Luo T, Piao M, et al. RIP1 and RIP3 contribute to shikonin-induced DNA double-strand breaks in glioma cells via increase of intracellular reactive oxygen species. Cancer Lett. 2017;390:77–90.
Qin X, Zhang L, Liu J, Lu Y, Zhou F, Jin F. Shikonin Induces Glioma Necroptosis, Stemness Decline, and Impedes (Immuno)Proteasome Activity. Stem Cells Int. 2024;2024:1348269.
Wang P, Zheng SY, Jiang RL, Wu HD, Li YA, Lu JL, et al. Necroptosis signaling and mitochondrial dysfunction cross-talking facilitate cell death mediated by chelerythrine in glioma. Free Radic Biol Med. 2023;202:76–96.
Zhou J, Li G, Han G, Feng S, Liu Y, Chen J, et al. Emodin induced necroptosis in the glioma cell line U251 via the TNF-α/RIP1/RIP3 pathway. Invest New Drugs. 2020;38:50–9.
Feng Y, Wang W, Zhang Y, Fu X, Ping K, Zhao J, et al. Synthesis and biological evaluation of celastrol derivatives as potential anti-glioma agents by activating RIP1/RIP3/MLKL pathway to induce necroptosis. Eur J Med Chem. 2022;229:114070.
Chakraborty S, Wei D, Tran M, Lang FF, Newman RA, Yang P. PBI-05204, a supercritical CO(2) extract of Nerium oleander, suppresses glioblastoma stem cells by inhibiting GRP78 and inducing programmed necroptotic cell death. Neoplasia. 2024;54:101008.
Yu J, Zhong B, Jin L, Hou Y, Ai N, Ge W, et al. 2-Methoxy-6-acetyl-7-methyljuglone (MAM) induced programmed necrosis in glioblastoma by targeting NAD(P)H: Quinone oxidoreductase 1 (NQO1). Free Radic Biol Med. 2020;152:336–47.
Clusmann J, Franco KC, Suárez DAC, Katona I, Minguez MG, Boersch N, et al. Acidosis induces RIPK1-dependent death of glioblastoma stem cells via acid-sensing ion channel 1a. Cell Death Dis. 2022;13:702.
Ishaq M, Ojha R, Sharma AP, Singh SK. Autophagy in cancer: Recent advances and future directions. Semin Cancer Biol. 2020;66:171–81.
Udristioiu A, Nica-Badea D. Autophagy dysfunctions associated with cancer cells and their therapeutic implications. Biomed Pharmacother. 2019;115:108892.
Rosenfeld MR, Ye X, Supko JG, Desideri S, Grossman SA, Brem S, et al. A phase I/II trial of hydroxychloroquine in conjunction with radiation therapy and concurrent and adjuvant temozolomide in patients with newly diagnosed glioblastoma multiforme. Autophagy. 2014;10:1359–68.
Compter I, Eekers DBP, Hoeben A, Rouschop KMA, Reymen B, Ackermans L, et al. Chloroquine combined with concurrent radiotherapy and temozolomide for newly diagnosed glioblastoma: a phase IB trial. Autophagy. 2021;17:2604–12.
Liu D, Zhu H, Cheng L, Li R, Ma X, Wang J, et al. Hypoxia-induced galectin-8 maintains stemness in glioma stem cells via autophagy regulation. Neuro Oncol. 2024;26:872–88.
Zeng L, Zheng W, Liu X, Zhou Y, Jin X, Xiao Y, et al. SDC1-TGM2-FLOT1-BHMT complex determines radiosensitivity of glioblastoma by influencing the fusion of autophagosomes with lysosomes. Theranostics. 2023;13:3725–43.
Li J, Sun Y, Zhao X, Ma Y, Xie Y, Liu S, et al. Radiation induces IRAK1 expression to promote radioresistance by suppressing autophagic cell death via decreasing the ubiquitination of PRDX1 in glioma cells. Cell Death Dis. 2023;14:259.
Yin HT, Hui L, Yang JH, Li Q, Li M, Zhao QQ, et al. Daurisoline suppress glioma progression by inhibiting autophagy through PI3K/AKT/mTOR pathway and increases TMZ sensitivity. Biochem Pharmacol. 2024;223:116113.
Acknowledgements
This work was supported by funding from the National Natural Science Foundation of China (No. 81473339), the Hunan Provincial Natural Science Foundation of China (No.2024JJ9532), and the Hunan Provincial Administration of Traditional Chinese Medicine Project (No. E2023026).
Author information
Authors and Affiliations
Contributions
Conceptualization, Yixiang Hu; Original Draft Preparation, Jincai Guo, Ya Liu, and Lijuan Zong; Visualization, figures, and tables, Lijuan Zong, Ying Huang, Jincai Guo, and Xiang Liu; Supervision, Yixiang Hu and Ya Liu.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, J., Zong, L., Huang, Y. et al. Unlocking glioma vulnerabilities: targeting regulated cell death pathways for innovative therapies. Cell Death Discov. (2026). https://doi.org/10.1038/s41420-026-02949-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-026-02949-8