Abstract
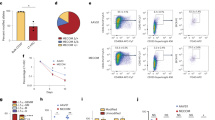
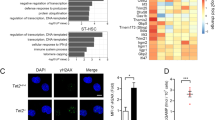
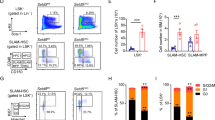
The histone H3 lysine 36 methyltransferase SETD2 is frequently mutated in various cancers, including leukemia. However, there has not been any functional model to show the contribution of SETD2 in hematopoiesis or the causal role of SETD2 mutation in tumorigenesis. In this study, using a conditional Setd2 knockout mouse model, we show that Setd2 deficiency skews hematopoietic differentiation and reduces the number of multipotent progenitors; although the number of phenotypic hematopoietic stem cells (HSCs) in Setd2-deleted mice is unchanged, functional assays, including serial BM transplantation, reveal that the self-renewal and competitiveness of HSCs are impaired. Intriguingly, Setd2-deleted HSCs, through a latency period, can acquire abilities to overcome the growth disadvantage and eventually give rise to hematopoietic malignancy characteristic of myelodysplastic syndrome. Gene expression profile of Setd2-deleted hematopoietic stem/progenitor cells (HSPCs) partially resembles that of Dnmt3a/Tet2 double knockout HSPCs, showing activation of the erythroid transcription factor Klf1-related pathway, which plays an important role in hematopoietic malignant transformation. Setd2 deficiency also induces DNA replication stress in HSCs, as reflected by an activated E2F gene regulatory network and repressed expression of the ribonucleotide reductase subunit Rrm2b, which results in proliferation and cell cycle abnormalities and genomic instability, allowing accumulation of secondary mutation(s) that synergistically contributes to tumorigenesis. Thus, our results demonstrate that Setd2 is required for HSC self-renewal, and provide evidence supporting the causal role of Setd2 deficiency in tumorigenesis. The underlying mechanism shall advance our understanding of epigenetic regulation of cancer and provide potential new therapeutic targets.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Rice, K. L., Hormaeche, I. & Licht, J. D. Epigenetic regulation of normal and malignant hematopoiesis. Oncogene 26, 6697–6714 (2007).
Cullen, S. M., Mayle, A., Rossi, L. & Goodell, M. A. Hematopoietic stem cell development: an epigenetic journey. Curr. Top. Dev. Biol. 107, 39–75 (2014).
Papaemmanuil, E. et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 374, 2209–2221 (2016).
Cancer Genome Atlas Research, N. et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 368, 2059–2074 (2013).
Bejar, R. et al. Clinical effect of point mutations in myelodysplastic syndromes. N. Engl. J. Med. 364, 2496–2506 (2011).
Rossi, L. et al. Less is more: unveiling the functional core of hematopoietic stem cells through knockout mice. Cell. Stem. Cell. 11, 302–317 (2012).
Wang, J. et al. Loss of Asxl1 leads to myelodysplastic syndrome-like disease in mice. Blood 123, 541–553 (2014).
Abdel-Wahab, O. et al. Deletion of Asxl1 results in myelodysplasia and severe developmental defects in vivo. J. Exp. Med. 210, 2641–2659 (2013).
Inoue, D. et al. Myelodysplastic syndromes are induced by histone methylation-altering ASXL1 mutations. J. Clin. Invest. 123, 4627–4640 (2013).
Dey, A. et al. Loss of the tumor suppressor BAP1 causes myeloid transformation. Science 337, 1541–1546 (2012).
Dameshek, W. Riddle: what do aplastic anemia, paroxysmal nocturnal hemoglobinuria (PNH) and “hypoplastic” leukemia have in common? Blood 30, 251–254 (1967).
Zhu, X. et al. Identification of functional cooperative mutations of SETD2 in human acute leukemia. Nat. Genet. 46, 287–293 (2014).
Mar, B. G. et al. Mutations in epigenetic regulators including SETD2 are gained during relapse in paediatric acute lymphoblastic leukaemia. Nat. Commun. 5, 3469 (2014).
Parker, H. et al. Genomic disruption of the histone methyltransferase SETD2 in chronic lymphocytic leukaemia. Leukemia 30, 2179–2186 (2016).
Zhang, J. et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature 481, 157–163 (2012).
Dalgliesh, G. L. et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature 463, 360–363 (2010).
Kandoth, C. et al. Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339 (2013).
Hu, M. et al. Histone H3 lysine 36 methyltransferase Hypb/Setd2 is required for embryonic vascular remodeling. Proc. Natl. Acad. Sci. USA 107, 2956–2961 (2010).
Sun, X. J. et al. Identification and characterization of a novel human histone H3 lysine 36-specific methyltransferase. J. Biol. Chem. 280, 35261–35271 (2005).
Edmunds, J. W., Mahadevan, L. C. & Clayton, A. L. Dynamic histone H3 methylation during gene induction: HYPB/Setd2 mediates all H3K36 trimethylation. Embo. J. 27, 406–420 (2008).
Baubec, T. et al. Genomic profiling of DNA methyltransferases reveals a role for DNMT3B in genic methylation. Nature 520, 243–247 (2015).
Morselli, M. et al. In vivo targeting of de novo DNA methylation by histone modifications in yeast and mouse. Elife 4, e06205 (2015).
Venkatesh, S. et al. Set2 methylation of histone H3 lysine 36 suppresses histone exchange on transcribed genes. Nature 489, 452–455 (2012).
Li, F. et al. The histone mark H3K36me3 regulates human DNA mismatch repair through its interaction with MutSalpha. Cell 153, 590–600 (2013).
Carrozza, M. J. et al. Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell 123, 581–592 (2005).
Neri, F. et al. Intragenic DNA methylation prevents spurious transcription initiation. Nature 543, 72–77 (2017).
Li, J., Moazed, D. & Gygi, S. P. Association of the histone methyltransferase Set2 with RNA polymerase II plays a role in transcription elongation. J. Biol. Chem. 277, 49383–49388 (2002).
Krogan, N. J. et al. Methylation of histone H3 by Set2 in Saccharomyces cerevisiae is linked to transcriptional elongation by RNA polymerase II. Mol. Cell. Biol. 23, 4207–4218 (2003).
Li, B., Howe, L., Anderson, S., Yates, J. R. 3rd & Workman, J. L. The Set2 histone methyltransferase functions through the phosphorylated carboxyl-terminal domain of RNA polymerase II. J. Biol. Chem. 278, 8897–8903 (2003).
Luco, R. F. et al. Regulation of alternative splicing by histone modifications. Science 327, 996–1000 (2010).
Park, I. Y. et al. Dual chromatin and cytoskeletal remodeling by SETD2. Cell 166, 950–962 (2016).
Zhang, Y. et al. H3K36 histone methyltransferase Setd2 is required for murine embryonic stem cell differentiation toward endoderm. Cell Rep. 8, 1989–2002 (2014).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Kanu, N. et al. SETD2 loss-of-function promotes renal cancer branched evolution through replication stress and impaired DNA repair. Oncogene 34, 5699–5708 (2015).
Pfister, S. X. et al. Inhibiting WEE1 selectively kills histone H3K36me3-deficient cancers by dNTP starvation. Cancer Cell. 28, 557–568 (2015).
Oguro, H., Ding, L. & Morrison, S. J. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell. Stem. Cell. 13, 102–116 (2013).
Kogan, S. C. et al. Bethesda proposals for classification of nonlymphoid hematopoietic neoplasms in mice. Blood 100, 238–245 (2002).
Li, Z. et al. Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood 118, 4509–4518 (2011).
Zhou, T., Kinney, M. C., Scott, L. M., Zinkel, S. S. & Rebel, V. I. Revisiting the case for genetically engineered mouse models in human myelodysplastic syndrome research. Blood 126, 1057–1068 (2015).
Santos, M. A. et al. DNA-damage-induced differentiation of leukaemic cells as an anti-cancer barrier. Nature 514, 107–111 (2014).
Tothova, Z. et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128, 325–339 (2007).
Sykes, S. M. et al. AKT/FOXO signaling enforces reversible differentiation blockade in myeloid leukemias. Cell 146, 697–708 (2011).
Takubo, K. et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell 7, 391–402 (2010).
Kong, L. J., Chang, J. T., Bild, A. H. & Nevins, J. R. Compensation and specificity of function within the E2F family. Oncogene 26, 321–327 (2007).
Nordlund, P. & Reichard, P. Ribonucleotide reductases. Annu. Rev. Biochem. 75, 681–706 (2006).
Tanaka, H. et al. A ribonucleotide reductase gene involved in a p53-dependent cell-cycle checkpoint for DNA damage. Nature 404, 42–49 (2000).
Bourdon, A. et al. Mutation of RRM2B, encoding p53-controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion. Nat. Genet. 39, 776–780 (2007).
Xu, L. et al. Genomic landscape of CD34+ hematopoietic cells in myelodysplastic syndrome and gene mutation profiles as prognostic markers. Proc. Natl Acad. Sci. USA 111, 8589–8594 (2014).
Pfeifer, G. P. et al. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene 21, 7435–7451 (2002).
Hatano, Y. et al. Molecular heterogeneity of the NUP98/HOXA9 fusion transcript in myelodysplastic syndromes associated with t(7;11)(p15; p15). Br. J. Haematol. 107, 600–604 (1999).
Pellagatti, A. et al. Gene expression profiles of CD34+ cells in myelodysplastic syndromes: involvement of interferon−stimulated genes and correlation to FAB subtype and karyotype. Blood 108, 337–345 (2006).
De Keersmaecker, K., Sulima, S. O. & Dinman, J. D. Ribosomopathies and the paradox of cellular hypo- to hyperproliferation. Blood 125, 1377–1382 (2015).
LaFave, L. M. et al. Loss of BAP1 function leads to EZH2-dependent transformation. Nat. Med. 21, 1344–1349 (2015).
Yildirim, E. et al. Xist RNA is a potent suppressor of hematologic cancer in mice. Cell 152, 727–742 (2013).
Nimer, S. D. Myelodysplastic syndromes. Blood 111, 4841–4851 (2008).
Della Porta, M. G. et al. Clinical relevance of bone marrow fibrosis and CD34-positive cell clusters in primary myelodysplastic syndromes. J. Clin. Oncol. 27, 754–762 (2009).
Tefferi, A. Myelofibrosis with myeloid metaplasia. N. Engl. J. Med. 342, 1255–1265 (2000).
Carvalho, S. et al. Histone methyltransferase SETD2 coordinates FACT recruitment with nucleosome dynamics during transcription. Nucleic Acids Res. 41, 2881–2893 (2013).
Acknowledgments
We thank Prof. Bo Zhou from Shanghai Institute of Biochemistry and Cell Biology for his constructive comments. We thank Shu-Min Xiong, Min Zhang, Jing Xie and other colleagues for their help and support. This work was supported by National Natural Science Foundation of China (81500080, 81670149); National Key Basic Research Project (973, 2013CB966801); 1000 Talents Program for Young Scholars; Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (20152506); and Samuel Waxman Cancer Research Foundation.
Author information
Authors and Affiliations
Contributions
Z.Y.L., H.Q.H., S.X.J., C.S.J., and C.Z. designed the experiments and wrote the manuscript. Z.Y.L., S.J.W., X.Y.Y., Z.Y., S.J.C., L.P., L.D., and X.A.N. performed all experiments. X.C.H. performed bioinformatics analysis. All authors were involved in the analysis and interpretation of the data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Zhang, YL., Sun, JW., Xie, YY. et al. Setd2 deficiency impairs hematopoietic stem cell self-renewal and causes malignant transformation. Cell Res 28, 476–490 (2018). https://doi.org/10.1038/s41422-018-0015-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41422-018-0015-9
This article is cited by
-
Pathogenesis and current status of the treatment of lung cancer associated with idiopathic pulmonary fibrosis
Respiratory Research (2025)
-
Epigenetic regulation of cancer stemness
Signal Transduction and Targeted Therapy (2025)
-
Setd2 ensures the establishment of a precise basal inflammatory state within murine hematopoietic stem/progenitor cells
Cell Death & Disease (2025)
-
Catalytic activity of Setd2 is essential for embryonic development in mice: establishment of a mouse model harboring patient-derived Setd2 mutation
Frontiers of Medicine (2024)
-
Tumor-suppressive functions of protein lysine methyltransferases
Experimental & Molecular Medicine (2023)