Abstract
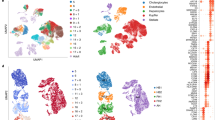
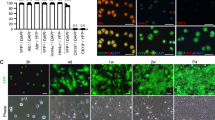
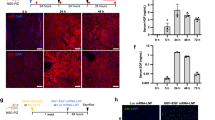
We report the generation of human ESC-derived, expandable hepatic organoids (hEHOs) using our newly established method with wholly defined (serum-free, feeder free) media. The hEHOs stably maintain phenotypic features of bipotential liver stem/progenitor cells that can differentiate into functional hepatocytes or cholangiocytes. The hEHOs can expand for 20 passages enabling large scale expansion to cell numbers requisite for industry or clinical programs. The cells from hEHOs display remarkable repopulation capacity in injured livers of FRG mice following transplantation, and they differentiate in vivo into mature hepatocytes. If implanted into the epididymal fat pads of immune-deficient mice, they do not generate non-hepatic lineages and have no tendency to form teratomas. We further develop a derivative model by incorporating human fetal liver mesenchymal cells (hFLMCs) into the hEHOs, referred to as hFLMC/hEHO, which can model alcoholic liver disease-associated pathophysiologic changes, including oxidative stress generation, steatosis, inflammatory mediators release and fibrosis, under ethanol treatment. Our work demonstrates that the hEHOs have considerable potential to be a novel, ex vivo pathophysiological model for studying alcoholic liver disease as well as a promising cellular source for treating human liver diseases.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Clevers, H. Modeling development and disease with organoids. Cell. 165, 1586–1597 (2016).
Sato, T. et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature. 459, 262 (2009).
Eiraku, M. et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 472, 51 (2011).
Lancaster, M. A. et al. Cerebral organoids model human brain development and microcephaly. Nature. 501, 373 (2013).
Takasato, M. et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 536, 238 (2016).
Huch, M. et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 32, 2708–2721 (2013).
Yamamoto, Y. et al. Long-term expansion of alveolar stem cells derived from human iPS cells in organoids. Nat. Methods. 14, 1097 (2017).
Boj Sylvia F. et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 160, 324–338 (2015).
Huch, M. et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 160, 299–312 (2015).
Hu, H. et al. Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell. 175, 1591–1606 e1519 (2018).
Ogawa, S. et al. Three-dimensional culture and cAMP signaling promote the maturation of human pluripotent stem cell-derived hepatocytes. Development. 140, 3285–3296 (2013).
Gieseck, R. L. 3rd et al. Maturation of induced pluripotent stem cell derived hepatocytes by 3D-culture. PloS one. 9, e86372–e86372 (2014).
Rashidi, H. et al. 3D human liver tissue from pluripotent stem cells displays stable phenotype in vitro and supports compromised liver function in vivo. Arch. Toxicol. 92, 3117–3129 (2018).
Takebe, T. et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 499, 481–484 (2013).
Guan Y., et al. Human hepatic organoids for the analysis of human genetic diseases. JCI Insight. 2, e94954 (2017).
Touboul, T. et al. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology. 51, 1754–1765 (2010).
Si-Tayeb, K. et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 51, 297–305 (2010).
Baxter, M. et al. Phenotypic and functional analyses show stem cell-derived hepatocyte-like cells better mimic fetal rather than adult hepatocytes. J. Hepatol. 62, 581–589 (2015).
Qin, J. et al. Connexin 32-mediated cell-cell communication is essential for hepatic differentiation from human embryonic stem cells. Sci. Rep. 6, 37388 (2016).
Huch, M. et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 494, 247–250 (2013).
Broutier, L. et al. Human primary liver cancer–derived organoid cultures for disease modeling and drug screening. Nat. Med. 23, 1424–1435 (2017).
Horslen, S. P. & Fox, I. J. Hepatocyte transplantation. Transplantation. 77, 1481–1486 (2004).
Hannan, N. R. et al. Generation of multipotent foregut stem cells from human pluripotent stem cells. Stem Cell Rep. 1, 293–306 (2013).
Ogawa, M. et al. Directed differentiation of cholangiocytes from human pluripotent stem cells. Nat. Biotechnol. 33, 853–861 (2015).
Avior, Y. et al. Microbial-derived lithocholic acid and vitamin K2 drive the metabolic maturation of pluripotent stem cells–derived and fetal hepatocytes. Hepatology. 62, 265–278 (2015).
Tanimizu, N., Miyajima, A. & Mostov, K. E. Liver progenitor cells develop cholangiocyte-type epithelial polarity in three-dimensional culture. Mol. Biol. Cell. 18, 1472–1479 (2007).
Sampaziotis, F. et al. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat. Biotechnol. 33, 845–852 (2015).
Azuma, H. et al. Robust expansion of human hepatocytes in Fah(−/−)/Rag2(−/−)/Il2rg (−/−) mice. Nat. Biotechnol. 25, 903–910 (2007).
Wang, Y. et al. Conversion of human gastric epithelial cells to multipotent endodermal progenitors using defined small molecules. Cell Stem Cell. 19, 449–461 (2016).
Yan, F. et al. Human embryonic stem cell-derived hepatoblasts are an optimal lineage stage for hepatitis C virus infection. Hepatology. 66, 717–735 (2017).
Benhamouche, S. et al. Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev. Cell. 10, 759–770 (2006).
Leite, S. B. et al. Novel human hepatic organoid model enables testing of drug-induced liver fibrosis in vitro. Biomaterials. 78, 1–10 (2016).
Coll, M. et al. Generation of hepatic stellate cells from human pluripotent stem cells enables in vitro modeling of liver fibrosis. Cell Stem Cell. 23, 101–113 e107 (2018).
Si-Tayeb, K., Lemaigre, F. P. & Duncan, S. A. Organogenesis and development of the liver. Dev. Cell. 18, 175–189 (2010).
Joshi, M. et al. Fetal liver-derived mesenchymal stromal cells augment engraftment of transplanted hepatocytes. Cytotherapy. 14, 657–669 (2012).
Henderson, N. C. et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat. Med. 19, 1617–1624 (2013).
Mederacke, I. et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 4, 2823 (2013).
Louvet, A. & Mathurin, P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 12, 231–242 (2015).
Liu J., et al. Liver extracellular matrices bioactivated hepatic spheroids as a model system for drug hepatotoxicity evaluations. Adv Biosyst 2, 1800110 (2018).
King, A. L. et al. Involvement of the mitochondrial permeability transition pore in chronic ethanol-mediated liver injury in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 306, G265–G277 (2014).
Osna, N. A., Donohue, T. M. & Kharbanda, K. K. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 38, 147–161 (2017).
Xu, M. J., Zhou, Z., Parker, R. & Gao, B. Targeting inflammation for the treatment of alcoholic liver disease. Pharm. Ther. 180, 77–89 (2017).
Petrasek, J. et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Investig. 122, 3476–3489 (2012).
Tilg, H., Moschen, A. R. & Szabo, G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 64, 955–965 (2016).
Lemmers, A. et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 49, 646–657 (2009).
Takebe, T. et al. Massive and reproducible production of liver buds entirely from human pluripotent stem cells. Cell Rep. 21, 2661–2670 (2017).
Avior, Y. et al. Microbial-derived lithocholic acid and vitamin K2 drive the metabolic maturation of pluripotent stem cells-derived and fetal hepatocytes. Hepatology. 62, 265–278 (2015).
Dolganiuc, A. & Szabo, G. In vitro and in vivo models of acute alcohol exposure. World J. Gastroenterol. 15, 1168–1177 (2009).
Jones, C. N. et al. Cultivating hepatocytes on printed arrays of HGF and BMP7 to characterize protective effects of these growth factors during in vitro alcohol injury. Biomaterials. 31, 5936–5944 (2010).
Lecluyse, E. L. & Alexandre, E. Isolation and culture of primary hepatocytes from resected human liver tissue. Methods Mol. Biol. 640, 57–82 (2010).
Broutier, L. et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 11, 1724 (2016).
Katsuda, T. et al. Conversion of terminally committed hepatocytes to culturable bipotent progenitor cells with regenerative capacity. Cell Stem Cell. 20, 41–55 (2017).
Li, B. et al. Adult mouse liver contains two distinct populations of cholangiocytes. Stem Cell Rep. 9, 478–489 (2017).
Donato, M. T., Jiménez, N., Castell, J. V. & Gómez-Lechón, M. J. Fluorescence-based assays for screening nine cytochrome P450 (P450) activities in intact cells expressing individual human P450 enzymes. Drug Metab. Dispos. 32, 699 (2004).
Acknowledgements
We thank Drs Zhigui Zeng, and Zhijun Zhu for their help with clinical samples; Drs Lola Reid and Xin Wang for critical review. Dr Xin Chang for TEM experiments; Mr Zhimin Li and Chuanwen Wang for bioinformatics analysis. This work was supported by the National Natural Science Foundations of China (No. 81730052), the Interdisciplinary Cooperation Project of Beijing Nova Program (Z1811100006218127), the National Major Scientific and Technological Special Project for “Significant New Drugs Development” (2018ZX09711003–001–002), the National Key Research and Development Program of China (No. 2016YFC1101305), the Science and Technology Planning Project of Guangdong China (2015A050502023), the Guangdong Province Science and Technology Program (2018KJYZ021) and Science and Technology Program of Guangzhou, China (STPG; 2016201604030054).
Author information
Authors and Affiliations
Contributions
Y.W. and S.W. conceived and designed the project. S.W. and X.W. conducted most of the experiments. Y.W., S.W., and X.W. wrote and edited the manuscript. Z.T., Y.S., and M.C. contributed to studies with cell culture, IF and tissue histology. J.L. helped with HCA experiments. F.Y. helped with in vivo transplantation experiments. J.C., T.C., C.L., and J.H. reviewed the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
These authors are co-senior authors: Jie Hu, Yunfang Wang
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, S., Wang, X., Tan, Z. et al. Human ESC-derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury. Cell Res 29, 1009–1026 (2019). https://doi.org/10.1038/s41422-019-0242-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41422-019-0242-8
This article is cited by
-
Advances in liver and pancreas organoids: how far we have come and where we go next
Nature Reviews Gastroenterology & Hepatology (2026)
-
Advances in liver organoids: replicating hepatic complexity for toxicity assessment and disease modeling
Stem Cell Research & Therapy (2025)
-
Single-cell sequencing and organoids: applications in organ development and disease
Molecular Biomedicine (2025)
-
Mesenchymal stem cell-derived extracellular vesicles attenuate periductal fibrosis by inhibiting Th17 differentiation in human liver multilineage organoids and Mdr2−/− mice
Journal of Nanobiotechnology (2025)
-
Dual-ligand engineered exosome regulates WNT signaling activation to promote liver repair and regeneration
Nature Communications (2025)