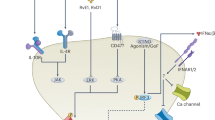
Abstract
Both opioids and nonsteroidal anti-inflammatory drugs (NSAIDS) produce deleterious side effects and fail to provide sustained relief in patients with chronic inflammatory pain. Peripheral neuroinflammation (PN) is critical for initiation and development of inflammatory pain. A better understanding of molecular mechanisms underlying PN would facilitate the discovery of new analgesic targets and the development of new therapeutics. Emerging evidence suggests that peripheral sensory neurons are not only responders to painful stimuli, but are also actively engaged in inflammation and immunity, whereas the intrinsic regulatory mechanism is poorly understood. Here we report the expression of proton-selective ion channel Hv1 in peripheral sensory neurons in rodents and humans, which was previously shown as selectively expressed in microglia in mammalian central nervous system. Neuronal Hv1 was up-regulated by PN or depolarizing stimulation, which in turn aggravates inflammation and nociception. Inhibiting neuronal Hv1 genetically or by a newly discovered selective inhibitor YHV98-4 reduced intracellular alkalization and ROS production in inflammatory pain, mitigated the imbalance in downstream SHP-1-pAKT signaling, and also diminished pro-inflammatory chemokine release to alleviate nociception and morphine-induced hyperalgesia and tolerance. Thus, our data reveal neuronal Hv1 as a novel target in analgesia strategy and managing opioids-related side effects.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Grosser, T., Woolf, C. J. & FitzGerald, G. A. Time for nonaddictive relief of pain. Science 355, 1026–1027 (2017).
Woolf, C. J. Capturing novel non-opioid pain targets. Biol. Psychiatry 87, 74–81 (2020).
Gregori, D. et al. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis a systematic review and meta-analysis. J. Am. Med. Assoc. 320, 2564–2579 (2018).
Busse, J. W. et al. Opioids for chronic noncancer pain a systematic review and meta-analysis. J. Am. Med. Assoc. 320, 2448–2460 (2018).
Ji, R. R., Chamessian, A. & Zhang, Y. Q. Pain regulation by non-neuronal cells and inflammation. Science 354, 572–577 (2016).
Donnelly, C. R. et al. STING controls nociception via type I interferon signalling in sensory neurons. Nature 591, 275–280 (2021).
Puig, S. & Gutstein, H. B. Opioids: keeping the good, eliminating the bad. Nat. Med. 23, 272–273 (2017).
Grace, P. M. et al. Nitroxidative signaling mechanisms in pathological pain. Trends Neurosci. 39, 862–879 (2016).
Wu, L. J. Voltage-gated proton channel Hv1 in microglia. Neuroscientist 20, 599–609 (2014).
Wu, L. J. et al. The voltage-gated proton channel Hv1 enhances brain damage from ischemic stroke. Nat. Neurosci. 15, 565–573 (2012).
Li, X. F. et al. Microglial Hv1 exacerbates secondary damage after spinal cord injury in mice. Biochem. Biophys. Res. Commun. 525, 208–215 (2020).
Li, X. F. et al. Deficiency of the microglial Hv1 proton channel attenuates neuronal pyroptosis and inhibits inflammatory reaction after spinal cord injury. J. Neuroinflammation 17, 263 (2020).
Murugan, M. et al. The voltage-gated proton channel Hv1 contributes to neuronal injury and motor deficits in a mouse model of spinal cord injury. Mol. Brain. 13, 143 (2020).
Li, Y. et al. The voltage-gated proton channel Hv1 plays a detrimental role in contusion spinal cord injury via extracellular acidosis-mediated neuroinflammation. Brain Behav. Immun. 91, 267–283 (2021).
De Simoni, A., Allen, N. J. & Attwell, D. Charge compensation for NADPH oxidase activity in microglia in rat brain slices does not involve a proton current. Eur. J. Neurosci. 28, 1146–1156 (2008).
Schilling, T. & Eder, C. Ion channel expression in resting and activated microglia of hippocampal slices from juvenile mice. Brain Res. 1186, 21–28 (2007).
Li, C. L. et al. Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res. 26, 83–102 (2016).
Usoskin, D. et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 18, 145–153 (2015).
Wang, K. K. et al. Single-cell transcriptomic analysis of somatosensory neurons uncovers temporal development of neuropathic pain. Cell Res. 31, 904–918 (2021).
Seredenina, T., Demaurex, N. & Krause, K. H. Voltage-gated proton channels as novel drug targets: from NADPH oxidase regulation to sperm biology. Antioxid. Redox Signal. 23, 490–513 (2015).
Cherny, V. V. & DeCoursey, T. E. pH-dependent inhibition of voltage-gated H(+) currents in rat alveolar epithelial cells by Zn(2+) and other divalent cations. J. Gen. Physiol. 114, 819–838 (1999).
Alabi, A. A., Bahamonde, M. I., Jung, H. J., Kim, J. I. & Swartz, K. J. Portability of paddle motif function and pharmacology in voltage sensors. Nature 450, 370–375 (2007).
Hong, L., Pathak, M. M., Kim, I. H., Ta, D. & Tombola, F. Voltage-sensing domain of voltage-gated proton channel Hv1 shares mechanism of block with pore domains. Neuron 77, 274–287 (2013).
Zhao, R. et al. Role of human Hv1 channels in sperm capacitation and white blood cell respiratory burst established by a designed peptide inhibitor. Proc. Natl. Acad. Sci. USA 115, E11847–E11856 (2018).
Li, Q. et al. Structural mechanism of voltage-dependent gating in an isolated voltage-sensing domain. Nat. Struct. Mol. Biol. 21, 244–252 (2014).
Takeshita, K. et al. X-ray crystal structure of voltage-gated proton channel. Nat. Struct. Mol. Biol. 21, 352–357 (2014).
Bayrhuber, M. et al. Nuclear magnetic resonance solution structure and functional behavior of the human proton channel. Biochemistry 58, 4017–4027 (2019).
Le Guilloux, V., Schmidtke, P. & Tuffery, P. Fpocket: an open source platform for ligand pocket detection. BMC Bioinformatics. 10, 168 (2009).
Hong, L., Kim, I. H. & Tombola, F. Molecular determinants of Hv1 proton channel inhibition by guanidine derivatives. Proc. Natl. Acad. Sci. USA 111, 9971–9976 (2014).
Boonamnaj, P. & Sompornpisut, P. Effect of ionization state on voltage-sensor structure in resting state of the Hv1 channel. J. Phys. Chem. B. 123, 2864–2873 (2019).
Wood, M. L. et al. Water wires in atomistic models of the Hv1 proton channel. Biochim. Biophys. Acta. 1818, 286–293 (2012).
Ramsey, I. S. et al. An aqueous H+ permeation pathway in the voltage-gated proton channel Hv1. Nat. Struct. Mol. Biol. 17, 869–875 (2010).
Gianti, E., Delemotte, L., Klein, M. L. & Carnevale, V. On the role of water density fluctuations in the inhibition of a proton channel. Proc. Natl. Acad. Sci. USA 113, E8359–E8368 (2016).
Geragotelis, A. D. et al. Voltage-dependent structural models of the human Hv1 proton channel from long-timescale molecular dynamics simulations. Proc. Natl. Acad. Sci. USA 117, 13490–13498 (2020).
Zaretzki, J., Matlock, M. & Swamidass, S. J. XenoSite: accurately predicting CYP-mediated sites of metabolism with neural networks. J. Chem. Inf. Model. 53, 3373–3383 (2013).
El Chemaly, A. et al. A voltage-activated proton current in human cardiac fibroblasts. Biochem. Biophys. Res. Commun. 340, 512–516 (2006).
Decher, T. et al. DCPIB is a novel selective blocker of I-Cl,I-swell and prevents swelling-induced shortening of guinea-pig atrial action potential duration. Br. J. Pharmacol. 134, 1467–1479 (2001).
Nilius, B., Sehrer, J. & Droogmans, G. Permeation properties and modulation of volume‐activated Cl−‐currents in human endothelial cells. Br. J. Pharmacol. 112, 1049–1056 (1994).
Shen, M. R. et al. Differential expression of volume-regulated anion channels during cell cycle progression of human cervical cancer cells. J. Physiol. 529, 385–394 (2000).
Ramsey, I. S., Moran, M. M., Chong, J. H. A. & Clapham, D. E. A voltage-gated proton-selective channel lacking the pore domain. Nature 440, 1213–1216 (2006).
Decoursey, T. E. Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 83, 475–579 (2003).
Byerly, L., Meech, R. & Moody, W. Jr. Rapidly activating hydrogen ion currents in perfused neurones of the snail, Lymnaea stagnalis. J. Physiol. 351, 199–216 (1984).
Fujita, F. et al. Intracellular alkalization causes pain sensation through activation of TRPA1 in mice. J. Clin. Invest. 118, 4049–4057 (2008).
Morgan, D. et al. Voltage-gated proton channels maintain pH in human neutrophils during phagocytosis. Proc. Natl. Acad. Sci. USA 106, 18022–18027 (2009).
Capasso, M. et al. HVCN1 modulates BCR signal strength via regulation of BCR-dependent generation of reactive oxygen species. Nat. Immunol. 11, 265–272 (2010).
Sisignano, M., Baron, R., Scholich, K. & Geisslinger, G. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat. Rev. Neurol. 10, 694–707 (2014).
Kim, H. K. et al. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain 111, 116–124 (2004).
Tappe-Theodor, A. & Kuner, R. Studying ongoing and spontaneous pain in rodents - challenges and opportunities. Eur. J. Neurosci. 39, 1881–1890 (2014).
Todd, P. A. & Sorkin, E. M. Diclofenac sodium. A reappraisal of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs 35, 244–285 (1988).
Zhou, Y. Q. et al. Reactive oxygen species scavengers ameliorate mechanical allodynia in a rat model of cancer-induced bone pain. Redox Biol. 14, 391–397 (2018).
Gwak, Y. S., Hassler, S. E. & Hulsebosch, C. E. Reactive oxygen species contribute to neuropathic pain and locomotor dysfunction via activation of CamKII in remote segments following spinal cord contusion injury in rats. Pain 154, 1699–1708 (2013).
Lu, J. M., Gong, N. A., Wang, Y. C. & Wang, Y. X. D-Amino acid oxidase-mediated increase in spinal hydrogen peroxide is mainly responsible for formalin-induced tonic pain. Br. J. Pharmacol. 165, 1941–1955 (2012).
Yoshizawa, K. et al. Antinociceptive activity of the novel RAGE inhibitor, papaverine, in a mouse model of chronic inflammatory pain. Synapse 75, e22188 (2021).
Schwartz, E. S., Lee, I., Chung, K. & Chung, J. M. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain 138, 514–524 (2008).
Chen, G. et al. PD-L1 inhibits acute and chronic pain by suppressing nociceptive neuron activity via PD-1. Nat. Neurosci. 20, 917–926 (2017).
Xiao, X. et al. Shp-1 dephosphorylates TRPV1 in dorsal root ganglion neurons and alleviates CFA-induced inflammatory pain in rats. Pain 156, 597–608 (2015).
Martin, L. J. et al. Epiregulin and EGFR interactions are involved in pain processing. J. Clin. Invest. 127, 3359–3372 (2017).
Chen, S. P. et al. PI3K/Akt pathway: a potential therapeutic target for chronic pain. Curr. Pharm. Des. 23, 1860–1868 (2017).
Zhang, Z. J., Jiang, B. C. & Gao, Y. J. Chemokines in neuron-glial cell interaction and pathogenesis of neuropathic pain. Cell. Mol. Life Sci. 74, 3275–3291 (2017).
Zhang, Z. J., Cao, D. L., Zhang, X., Ji, R. R. & Gao, Y. J. Chemokine contribution to neuropathic pain: Respective induction of CXCL1 and CXCR2 in spinal cord astrocytes and neurons. Pain 154, 2185–2197 (2013).
Lin, C. P. et al. Role of spinal CXCL1 (GRO alpha) in opioid tolerance a human-to-rodent translational study. Anesthesiology 122, 666–676 (2015).
Corder, G. et al. Loss of μ opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nat. Med. 23, 164–173 (2017).
Kornilov, P., Peretz, A. & Attali, B. Channel gating pore: a new therapeutic target. Cell Res. 23, 1067–1068 (2013).
Peretz, A. et al. Targeting the voltage sensor of Kv7.2 voltage-gated K+ channels with a new gating-modifier. Proc. Natl. Acad. Sci. USA 107, 15637–15642 (2010).
Ottosson, N. E. et al. A drug pocket at the lipid bilayer-potassium channel interface. Sci. Adv. 3, e1701099 (2017).
Marvaldi, L. et al. Importin alpha 3 regulates chronic pain pathways in peripheral sensory neurons. Science 369, 842–846 (2020).
Ma, Y. Q. et al. Discovery of an inhibitor for the TREK-1 channel targeting an intermediate transition state of channel gating. J. Med. Chem. 63, 10972–10983 (2020).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011).
Robert, X. & Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42, W320–W324 (2014).
Okamura, Y., Fujiwara, Y. & Sakata, S. Gating mechanisms of voltage-gated proton channels. Annu. Rev. Biochem. 84, 685–709 (2015).
Eswar, N. et al. Comparative protein structure modeling using Modeller. Curr. Protoc. Bioinformatics 15, 5.6. 1–5.6. 30 (2006).
Friesner, R. A. et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 47, 1739–1749 (2004).
Pronk, S. et al. GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 29, 845–854 (2013).
Pastor, R. W. & MacKerell, A. D. Development of the CHARMM force field for lipids. J. Phys. Chem. Lett. 2, 1526–1532 (2011).
Huang, J. & MacKerell, A. D. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 34, 2135–2145 (2013).
Vanommeslaeghe, K. et al. CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 31, 671–690 (2010).
Kumari, R., Kumar, R., Lynn, A., & Consort, O. S. D. D. g_mmpbsa-A GROMACS Tool for High-Throughput MM-PBSA Calculations. J. Chem. Inf. Model. 54, 1951–1962 (2014).
Zhao, X. L. et al. A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat. Neurosci. 16, 1024–1031 (2013).
Liao, P. et al. Selective activation of TWIK-related acid-sensitive K+ 3 subunit-containing channels is analgesic in rodent models. Sci. Transl. Med. 11, eaaw8434 (2019).
Ferreira, J., Triches, K. M., Medeiros, R. & Calixto, J. B. Mechanisms involved in the nociception produced by peripheral protein kinase c activation in mice. Pain 117, 171–181 (2005).
Takeshita, H. et al. Modified forelimb grip strength test detects aging-associated physiological decline in skeletal muscle function in male mice. Sci. Rep. 7, 42323 (2017).
Wang, Z. L. et al. Anti-PD-1 treatment impairs opioid antinociception in rodents and nonhuman primates. Sci. Transl. Med. 12, eaaw6471 (2020).
Geis, C., Geuss, E., Sommer, C., Schmidt, H. H. H. W. & Kleinschnitz, C. NOX4 is an early initiator of neuropathic pain. Exp. Neurol. 288, 94–103 (2017).
Ibi, M. et al. Involvement of NOX1/NADPH oxidase in morphine-induced analgesia and tolerance. J. Neurosci. 31, 18094–18103 (2011).
Kim, U. J., Won, R. & Lee, K. H. Neuroprotective effects of okadaic acid following oxidative injury in organotypic hippocampal slice culture. Brain Res. 1618, 241–248 (2015).
Kim, H. A., Lee, K. H. & Lee, B. H. Neuroprotective effect of melatonin against kainic acid-induced oxidative injury in hippocampal slice culture of rats. Int. J. Mol. Sci. 15, 5940–5951 (2014).
Yamamoto, T., Takagawa, S., Katayama, I., Mizushima, Y. & Nishioka, K. Effect of superoxide dismutase on bleomycin-induced dermal sclerosis: Implications for the treatment of systemic sclerosis. J. Invest. Dermatol. 113, 843–847 (1999).
Riquelme, E. et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 178, 795–806 (2019).
Acknowledgements
We thank Prof. Jun-Li Cao (Xuzhou Medical University, China), Prof. Xia Zhang (University of Ottawa, Canada) for helpful discussion on the manuscript, J. Zhang, Z. Yang, and L. Bai (Histology and Imaging platform, Core Facility of West China Hospital) for assistance with acquiring some ISH images; Li Li, Fei Chen and Chunjuan Bao (Institute of Clinical Pathology, West China Hospital) for processing histological staining. Yuandong Liu and Prof. Yang Tian (East China Normal University) for assistance in pH imaging. We thank the support of ECNU Multifunctional Platform for Innovation (001 and 011). This work was equally funded by the National Natural Science Foundation of China (32071003 to R.J.) and the Ministry of Science and Technology of China (2018ZX09711002 to Q.Z.), partially funded by the National Natural Science Foundation of China (81873808 to Y.Z, 31600832 to R.J., 31800699 to Q.Z.), the Fundamental Research Funds for the Central Universities (to H.Y. and 2018SCUH0086 to R.J.), the “XingFuZhiHua” funding of ECNU (44300-19311-542500/006 to H.Y.), the Department of Science and Technology of Sichuan Province (2020ZYD006 to R.J.) and the 1-3-5 Project for Disciplines of Excellence of West China Hospital of Sichuan University (ZYJC21034 to R.J.).
Author information
Authors and Affiliations
Contributions
Q.Z. performed drug design and computations. Y.R. performed electrophysiology in DRG, DRG microinjection, behavioral tests, cytokine and chemokine measurement, ISH, IHC, and ROS imaging. Y.M performed mouse genetics on Hvcn1−/− mice, pharmacokinetics study, behavioral tests, skin histopathological study, and ROS imaging. P.G. performed electrophysiology in HEK293 cells. P.L. performed ISH, IHC, pH and ROS imaging. Y.Luo performed ISH, IHC, ROS imaging, ELISA and behavioral tests. J.M. performed IHC and pH imaging. Z.C. and J.F. performed electrophysiology in HEK293 cells. Y.Zhang performed IHC imaging. Y.Li performed ROS imaging. L.Y. and D.L. performed some behavioral tests. J.S. performed drug design. W.H. and X.X. acquired and prepared human skin tissues. Y.G. performed behavioral tests, and ROS imaging. L.M. synthetized YHV98-4. Y.Zuo and J.L. oversaw some behavioral tests. H.Y. and R.J. initiated, supervised the project, analyzed the experiments, and wrote the manuscript with input from all coauthors.
Corresponding authors
Ethics declarations
Competing interests
H.Y., R.J., and Q.Z. are inventors on a patent application (202011283354.3) submitted by East China Normal University, West China Hospital of Sichuan University and Shaoxing ZeroIn Biomedicines Co. Ltd. that cover the potential usage of YHV98-1, YHV98-4 and their derivatives.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, Q., Ren, Y., Mo, Y. et al. Inhibiting Hv1 channel in peripheral sensory neurons attenuates chronic inflammatory pain and opioid side effects. Cell Res 32, 461–476 (2022). https://doi.org/10.1038/s41422-022-00616-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41422-022-00616-y
This article is cited by
-
Brain targeted lipid nanoparticles with Hv1 inhibitors alleviate neuroinflammation post-ischemic stroke
Journal of Nanobiotechnology (2025)
-
C6 peptide blockade of Hv1 channels inhibits neutrophil migration into the lungs to suppress Pseudomonas aeruginosa-induced acute lung injury
Respiratory Research (2025)
-
Hv1 inhibition rescues AD pathology by restoring microglial mitochondrial function and enhancing mitochondrial transfer
Experimental & Molecular Medicine (2025)
-
Dermorphin [D-Arg2, Lys4] (1–4) Amide Attenuates Burn Pain by Inhibiting TRPV1/NR2B Mediated Neuroinflammatory Signalling
Molecular Neurobiology (2025)
-
A small-molecule activation mechanism that directly opens the KCNQ2 channel
Nature Chemical Biology (2024)