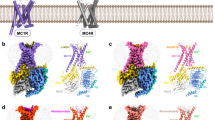
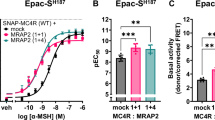
Abstract
G protein-coupled receptors (GPCRs) are regulated by various downstream proteins, of which the melanocortin receptor accessory protein 1 (MRAP1) is closely involved in the regulation of melanocortin receptor 2 (MC2R). Assisted by MRAP1, MC2R responds to adrenocorticotropic hormone (ACTH) and stimulates glucocorticoid biogenesis and cortisol secretion. MC2R activation plays an essential role in the hypothalamic-pituitary-adrenal (HPA) axis that regulates stress response, while its dysfunction causes glucocorticoid insufficiency- or cortisol excess-associated disorders. Here, we present a cryo-electron microscopy (cryo-EM) structure of the ACTH-bound MC2R–Gs–MRAP1 complex. Our structure, together with mutagenesis analysis, reveals a unique sharp kink at the extracellular region of MRAP1 and the ‘seat-belt’ effect of MRAP1 on stabilizing ACTH binding and MC2R activation. Mechanisms of ACTH recognition by MC2R and receptor activation are also demonstrated. These findings deepen our understanding of GPCR regulation by accessory proteins and provide valuable insights into the ab initio design of therapeutic agents targeting MC2R.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The corresponding coordinates and cryo-EM density map have been deposited in the Protein Data Bank (http://www.rcsb.org/pdb) with code 8GY7, and in EMDB (http://www.ebi.ac.uk/pdbe/emdb/) with code EMD-34371, respectively.
References
Cooray, S. N. & Clark, A. J. L. Melanocortin receptors and their accessory proteins. Mol. Cell Endocrinol. 331, 215–221 (2011).
Webb, T. R. & Clark, A. J. L. Minireview: the melanocortin 2 receptor accessory proteins. Mol. Endocrinol. 24, 475–484 (2010).
Rouault, A. A. J., Srinivasan, D. K., Yin, T. C., Lee, A. A. & Sebag, J. A. Melanocortin Receptor Accessory Proteins (MRAPs): Functions in the melanocortin system and beyond. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 2462–2467 (2017).
Sebag, J. A. & Hinkle, P. M. Regions of Melanocortin 2 (MC2) receptor accessory protein necessary for dual topology and MC2 receptor trafficking and signaling. J. Biol. Chem. 284, 610–618 (2009).
Roy, S. et al. Mechanisms of melanocortin-2 receptor (MC2R) internalization and recycling in human embryonic kidney (hek) cells: identification of Key Ser/Thr (S/T) amino acids. Mol. Endocrinol. 25, 1961–1977 (2011).
Roy, S., Rached, M. & Gallo-Payet, N. Differential regulation of the human adrenocorticotropin receptor [melanocortin-2 receptor (MC2R)] by human MC2R accessory protein isoforms alpha and beta in isogenic human embryonic kidney 293 cells. Mol. Endocrinol. 21, 1656–1669 (2007).
Hinkle, P. M. & Sebag, J. A. Structure and function of the melanocortin2 receptor accessory protein (MRAP). Mol. Cell Endocrinol. 300, 25–31 (2009).
Malik, S., Dolan, T. M., Maben, Z. J. & Hinkle, P. M. Adrenocorticotropic Hormone (ACTH) responses require actions of the melanocortin-2 receptor accessory protein on the extracellular surface of the plasma membrane. J. Biol. Chem. 290, 27972–27985 (2015).
Sebag, J. A. & Hinkle, P. M. Melanocortin-2 receptor accessory protein MRAP forms antiparallel homodimers. Proc. Natl. Acad. Sci. USA 104, 20244–20249 (2007).
Maben, Z. J., Malik, S., Jiang, L. Y. H. & Hinkle, P. M. Dual topology of the melanocortin-2 receptor accessory protein is stable. Front. Endocrinol. 7, 96 (2016).
Heyder, N. A. et al. Structures of active melanocortin-4 receptor-Gs-protein complexes with NDP-alpha-MSH and setmelanotide. Cell Res. 31, 1176–1189 (2021).
Mountjoy, K. G., Robbins, L. S., Mortrud, M. T. & Cone, R. D. The cloning of a family of genes that encode the melanocortin receptors. Science 257, 1248–1251 (1992).
Schwyzer, R. ACTH: a short introductory review. Ann. N. Y. Acad. Sci. 297, 3–26 (1977).
Yang, Y. & Harmon, C. M. Molecular signatures of human melanocortin receptors for ligand binding and signaling. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 2436–2447 (2017).
Yang, Y. K. & Harmon, C. M. Molecular determinants of ACTH receptor for ligand selectivity. Mol. Cell Endocrinol. 503, 110688 (2020).
Cawley, N. X., Li, Z. & Loh, Y. P. 60 YEARS OF POMC: Biosynthesis, trafficking, and secretion of pro-opiomelanocortin-derived peptides. J. Mol. Endocrinol. 56, T77–T97 (2016).
Liang, L., Angleson, J. K. & Dores, R. M. Using the human melanocortin-2 receptor as a model for analyzing hormone/receptor interactions between a mammalian MC2 receptor and ACTH(1-24). Gen. Comp. Endocr. 181, 203–210 (2013).
Dores, R. M. & Liang, L. Analyzing the activation of the melanocortin-2 receptor of tetrapods. Gen. Comp. Endocr. 203, 3–9 (2014).
Kim, J. D., Leyva, S. & Diano, S. Hormonal regulation of the hypothalamic melanocortin system. Front. Physiol. 5, 480 (2014).
Russell, G. & Lightman, S. The human stress response. Nat. Rev. Endocrinol. 15, 525–534 (2019).
Chung, T. T., Chan, L. F., Metherell, L. A. & Clark, A. J. Phenotypic characteristics of familial glucocorticoid deficiency (FGD) type 1 and 2. Clin. Endocrinol. (Oxf) 72, 589–594 (2010).
Heshmatzad, K., Mahdieh, N., Rabbani, A., Didban, A. & Rabbani, B. The genetic perspective of familial glucocorticoid deficiency: in silico analysis of two novel variants. Int. J. Endocrinol. 2020, 2190508 (2020).
Meimaridou, E. et al. Familial glucocorticoid deficiency: new genes and mechanisms. Mol. Cell Endocrinol. 371, 195–200 (2013).
Metherell, L. A. et al. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat. Genet. 37, 166–170 (2005).
Chan, L. F., Metherell, L. A. & Clark, A. J. Effects of melanocortins on adrenal gland physiology. Eur. J. Pharmacol. 660, 171–180 (2011).
Ghaddhab, C., Vuissoz, J. M. & Deladoey, J. From bioinactive ACTH to ACTH antagonist: the clinical perspective. Front. Endocrinol. 8, 17 (2017).
Goldenberg, A. J. et al. Effect of a melanocortin type 2 receptor (MC2R) antagonist on the corticosterone response to hypoxia and ACTH stimulation in the neonatal rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315, R128–R133 (2018).
Bouw, E. et al. Development of potent selective competitive-antagonists of the melanocortin type 2 receptor. Mol. Cell Endocrinol. 394, 99–104 (2014).
Yu, J. et al. Determination of the melanocortin-4 receptor structure identifies Ca2+ as a cofactor for ligand binding. Science 368, 428–433 (2020).
Israeli, H. et al. Structure reveals the activation mechanism of the MC4 receptor to initiate satiation signaling. Science 372, 808–814 (2021).
Ma, S. S. et al. Structural mechanism of calcium-mediated hormone recognition and G beta interaction by the human melanocortin-1 receptor. Cell Res. 31, 1061–1071 (2021).
Zhang, H. B. et al. Structural insights into ligand recognition and activation of the melanocortin-4 receptor. Cell Res. 31, 1163–1175 (2021).
Berruien, N. N. A. & Smith, C. L. Emerging roles of melanocortin receptor accessory proteins (MRAP and MRAP2) in physiology and pathophysiology. Gene 757, 144949 (2020).
Hay, D. L. & Pioszak, A. A. Receptor activity-modifying proteins (RAMPs): new insights and roles. Annu. Rev. Pharmacol. Toxicol. 56, 469–487 (2016).
Liang, Y. L. et al. Cryo-EM structure of the active, Gs-protein complexed, human CGRP receptor. Nature 561, 492–497 (2018).
Josephs, T. M. et al. Structure and dynamics of the CGRP receptor in apo and peptide-bound forms. Science 372, eabf7258 (2021).
Liang, Y. L. et al. Structure and dynamics of adrenomedullin receptors AM1 and AM2 reveal key mechanisms in the control of receptor phenotype by receptor activity-modifying proteins. ACS Pharmacol. Transl. Sci. 3, 263–284 (2020).
Cao, J. et al. A structural basis for amylin receptor phenotype. Science 375, eabm9609 (2022).
Nunez Miguel, R., Sanders, J., Furmaniak, J. & Smith, B. R. Structure and activation of the TSH receptor transmembrane domain. Auto Immun. Highlights 8, 2 (2017).
Duan, J. et al. Structures of full-length glycoprotein hormone receptor signalling complexes. Nature 598, 688–692 (2021).
Carpenter, B., Nehme, R., Warne, T., Leslie, A. G. & Tate, C. G. Structure of the adenosine A(2A) receptor bound to an engineered G protein. Nature 536, 104–107 (2016).
Kimanius, D., Dong, L., Sharov, G., Nakane, T. & Scheres, S. H. W. New tools for automated cryo-EM single-particle analysis in RELION-4.0. Biochem. J. 478, 4169–4185 (2021).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Sanchez-Garcia, R. et al. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun. Biol. 4, 874 (2021).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Acknowledgements
The cryo-EM data of the ACTH–MC2R–Gs–MRAP1 complex were collected at the Advanced Center for Electron Microscopy, Shanghai Institute of Materia Medica (SIMM). We thank all the staff at the cryo-EM facilities for their technical support. This work was partially supported by the National Natural Science Foundation (32171187 to Y.J., 82121005 to Y.J. and H.E.X., 32130022 to H.E.X., 81872915 to M.-W.W., 81773792 and 81973373 to D.Y.); the Ministry of Science and Technology (China) grants (2018YFA0507002 to H.E.X. and M.-W.W.); the Shanghai Municipal Science and Technology Major Project (2019SHZDZX02 to H.E.X.); Shanghai Municipal Science and Technology Major Project (H.E.X.); the CAS Strategic Priority Research Program (XDB37030103 to H.E.X.).
Author information
Authors and Affiliations
Contributions
P.L. designed the expression constructs, purified the MC2R complex protein, prepared the final samples for negative stain and cryo-EM data collection, conducted functional studies, and prepared figures and manuscript draft; W.F. conducted functional studies with the help of A.D., X. Chen, and X. Cai, supervised by M.-W.W. and D.Y.; S.M. participated in the preparation of constructs and expression screening; K.W. and Q.Y. participated in cryo-EM data acquisition; M.-W.W. participated in manuscript preparation; Y.J. and H.E.X. conceived and supervised the project, analyzed the structures, and wrote the manuscript with inputs from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, P., Feng, W., Ma, S. et al. Structural basis of signaling regulation of the human melanocortin-2 receptor by MRAP1. Cell Res 33, 46–54 (2023). https://doi.org/10.1038/s41422-022-00751-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41422-022-00751-6
This article is cited by
-
The molecular basis of μ-opioid receptor signaling plasticity
Cell Research (2025)
-
MRAP2 modifies the signaling and oligomerization state of the melanocortin-4 receptor
Nature Communications (2025)
-
G protein-coupled receptors (GPCRs): advances in structures, mechanisms and drug discovery
Signal Transduction and Targeted Therapy (2024)
-
Synthesis and Pharmacological Characterization of Adrenocorticotropic Hormone (ACTH 1–24) and C-Terminal Truncated Analogues Identifies the Minimal ACTH N-Terminal Fragment Required for Melanocorton-2 Receptor Activation
International Journal of Peptide Research and Therapeutics (2024)
-
Buckle up! How the nano-seatbelt MRAP1 fastens ACTH in its orthosteric seat
Cell Research (2023)