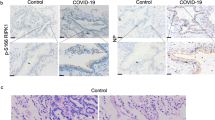
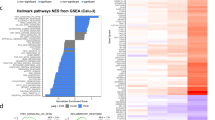
Abstract
SARS-CoV-2 infection can trigger strong inflammatory responses and cause severe lung damage in COVID-19 patients with critical illness. However, the molecular mechanisms by which the infection induces excessive inflammatory responses are not fully understood. Here, we report that SARS-CoV-2 infection results in the formation of viral Z-RNA in the cytoplasm of infected cells and thereby activates the ZBP1-RIPK3 pathway. Pharmacological inhibition of RIPK3 by GSK872 or genetic deletion of MLKL reduced SARS-CoV-2-induced IL-1β release. ZBP1 or RIPK3 deficiency leads to reduced production of both inflammatory cytokines and chemokines during SARS-CoV-2 infection both in vitro and in vivo. Furthermore, deletion of ZBP1 or RIPK3 alleviated SARS-CoV-2 infection-induced immune cell infiltration and lung damage in infected mouse models. These results suggest that the ZBP1-RIPK3 pathway plays a critical role in SARS-CoV-2-induced inflammatory responses and lung damage. Our study provides novel insights into how SARS-CoV-2 infection triggers inflammatory responses and lung pathology, and implicates the therapeutic potential of targeting ZBP1-RIPK3 axis in treating COVID-19.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hu, B., Guo, H., Zhou, P. & Shi, Z. L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 19, 141–154 (2021).
Wiersinga, W. J., Rhodes, A., Cheng, A. C., Peacock, S. J. & Prescott, H. C. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus disease 2019 (COVID-19): a review. JAMA 324, 782–793 (2020).
Li, H. et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet 395, 1517–1520 (2020).
Tay, M. Z., Poh, C. M., Renia, L., MacAry, P. A. & Ng, L. F. P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 20, 363–374 (2020).
Mehta, P. et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395, 1033–1034 (2020).
Angus, D. C. et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA 324, 1317–1329 (2020).
Dequin, P. F. et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA 324, 1298–1306 (2020).
Sterne, J. A. C. et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 324, 1330–1341 (2020).
Horby, P. et al. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 384, 693–704 (2021).
Tomazini, B. M. et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA 324, 1307–1316 (2020).
Nailwal, H. & Chan, F. K. Necroptosis in anti-viral inflammation. Cell Death Differ. 26, 4–13 (2019).
Verdonck, S., Nemegeer, J., Vandenabeele, P. & Maelfait, J. Viral manipulation of host cell necroptosis and pyroptosis. Trends Microbiol. 6, 593–605 (2021).
Chen, D. et al. RIP3-dependent necroptosis contributes to the pathogenesis of chronic obstructive pulmonary disease. JCI Insight 6, e144689 (2021).
Koehler, H. et al. Vaccinia virus E3 prevents sensing of Z-RNA to block ZBP1-dependent necroptosis. Cell Host Microbe 29, 1266–1276.e5 (2021).
Moriwaki, K. et al. The necroptosis adaptor RIPK3 promotes injury-induced cytokine expression and tissue repair. Immunity 41, 567–578 (2014).
Zheng, M. & Kanneganti, T. D. The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis). Immunol. Rev. 297, 26–38 (2020).
Zhang, T. et al. Influenza virus Z-RNAs induce ZBP1-mediated necroptosis. Cell 180, 1115–1129.e13 (2020).
Balachandran, S. & Mocarski, E. S. Viral Z-RNA triggers ZBP1-dependent cell death. Curr. Opin. Virol. 51, 134–140 (2021).
Li, Y. et al. SARS-CoV-2 induces double-stranded RNA-mediated innate immune responses in respiratory epithelial-derived cells and cardiomyocytes. Proc. Natl. Acad. Sci. USA 118, e2022643118 (2021).
Lin, J. W. et al. Genomic monitoring of SARS-CoV-2 uncovers an Nsp1 deletion variant that modulates type I interferon response. Cell Host Microbe 29, 489–502.e8 (2021).
Mahoney, M. et al. A novel class of TMPRSS2 inhibitors potently block SARS-CoV-2 and MERS-CoV viral entry and protect human epithelial lung cells. Proc. Natl. Acad. Sci. USA 118, e2108728118 (2021).
Christgen, S. et al. Identification of the PANoptosome: a molecular platform triggering pyroptosis, apoptosis, and necroptosis (PANoptosis). Front. Cell. Infect. Microbiol. 10, 237 (2020).
Li, S. et al. SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduct. Target. Ther. 5, 235 (2020).
Takaoka, A. et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448, 501–505 (2007).
Upton, J. W., Kaiser, W. J. & Mocarski, E. S. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 11, 290–297 (2012).
Sun, J. et al. Generation of a broadly useful model for COVID-19 pathogenesis, vaccination, and treatment. Cell 182, 734–743.e5 (2020).
Jiao, H. et al. Z-nucleic-acid sensing triggers ZBP1-dependent necroptosis and inflammation. Nature 580, 391–395 (2020).
Rebsamen, M. et al. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-kappaB. EMBO Rep. 10, 916–922 (2009).
Huang, K. et al. Q493K and Q498H substitutions in spike promote adaptation of SARS-CoV-2 in mice. EBioMedicine 67, 103381 (2021).
Zheng, H. Y. et al. Pro-inflammatory microenvironment and systemic accumulation of CXCR3+ cell exacerbate lung pathology of old rhesus macaques infected with SARS-CoV-2. Signal Transduct. Target. Ther. 6, 328 (2021).
Desai, N. et al. Temporal and spatial heterogeneity of host response to SARS-CoV-2 pulmonary infection. Nat. Commun. 11, 6319 (2020).
Nienhold, R. et al. Two distinct immunopathological profiles in autopsy lungs of COVID-19. Nat. Commun. 11, 5086 (2020).
Harris, T. H. et al. Generalized Levy walks and the role of chemokines in migration of effector CD8+ T cells. Nature 486, 545–548 (2012).
Qian, B. Z. et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225 (2011).
Martens, S., Hofmans, S., Declercq, W., Augustyns, K. & Vandenabeele, P. Inhibitors targeting RIPK1/RIPK3: old and new drugs. Trends Pharmacol. Sci. 41, 209–224 (2020).
Silke, J., Rickard, J. A. & Gerlic, M. The diverse role of RIP kinases in necroptosis and inflammation. Nat. Immunol. 16, 689–697 (2015).
Peng, R. et al. Human ZBP1 induces cell death-independent inflammatory signaling via RIPK3 and RIPK1. EMBO Rep. e55839 (2022).
Cho, Y. S. et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009).
Carpenter, E. A., Ruby, J. & Ramshaw, I. A. IFN-gamma, TNF, and IL-6 production by vaccinia virus immune spleen cells. An in vitro study. J. Immunol. 152, 2652–2659 (1994).
Polykratis, A. et al. Cutting edge: RIPK1 Kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J. Immunol. 193, 1539–1543 (2014).
Liu, Z. et al. A class of viral inducer of degradation of the necroptosis adaptor RIPK3 regulates virus-induced inflammation. Immunity 54, 247–258.e7 (2021).
Xu, G. et al. SARS-CoV-2 promotes RIPK1 activation to facilitate viral propagation. Cell Res. 31, 1230–1243 (2021).
Zhou, P. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020).
Acknowledgements
This work was supported by the National Key R&D Program of China (2018YFA0507201, 2021YFC2300702, 2022YFC2303302), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB29010204), Creative Research Group Program of Natural Science Foundation of Hubei Province (2022CFA021), the National Natural Science Foundation of China (32070179), Self-supporting Program of Guangzhou Laboratory (SRPG22-001), the Hundred Talents Program of Chinese Academy of Sciences (to Ke Peng), and the Advanced Customer Cultivation Project of Wuhan National Biosafety Laboratory, Chinese Academy of Sciences (2022ACCP-MS10). We would like to thank Dr Ding Gao, Ms Anna Du and Ms Juan Min, from the Center for Instrumental Analysis and Metrology, Wuhan Institute of Virology, Chinese Academy of Science for technical assistance. We would like to acknowledge Ms Xuefang An, Mr He Zhao, Ms Li Li and Ms Youling Zhu from the Center for Experimental Animals, Wuhan Institute of Virology for help in animal experiments. We thank Tao Du, Jia Wu, Hao Tang and Jun Liu from the BSL-3 Laboratory, Wuhan Institute of Virology for their essential support.
Author information
Authors and Affiliations
Contributions
K.P., P.Z. and B.L. conceived and supervised the study. K.P., P.Z., B.L., S.L., Y.Z. and Z.G. participated in the study design, analyzed the data, and wrote the manuscript. S.L., Y.Z., Z.G., M.D.Y., H.L., M.M.Y., C.Z. and Z.Z. performed the experiments. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, S., Zhang, Y., Guan, Z. et al. SARS-CoV-2 Z-RNA activates the ZBP1-RIPK3 pathway to promote virus-induced inflammatory responses. Cell Res 33, 201–214 (2023). https://doi.org/10.1038/s41422-022-00775-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41422-022-00775-y
This article is cited by
-
Inflammatory microglia signals drive A1-like polarization of astrocytes even in the presence of HIV-1 Tat
Molecular Neurobiology (2026)
-
Pattern recognition receptors: function, regulation and therapeutic potential
Signal Transduction and Targeted Therapy (2025)
-
Soluble tissue factor generated by necroptosis-triggered shedding is responsible for thrombosis
Cell Research (2025)
-
Acute SARS-CoV-2 infection
Nature Reviews Disease Primers (2025)
-
Necroptosis in obesity: a complex cell death event
Apoptosis (2025)