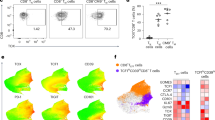
Abstract
Acute viral infection causes illness and death. In addition, an infection often results in increased susceptibility to a secondary infection, but the mechanisms behind this susceptibility are poorly understood. Since its initial identification as a marker for resident memory CD8+ T cells in barrier tissues, the function and regulation of CD103 integrin (encoded by ITGAE gene) have been extensively investigated. Nonetheless, the function and regulation of the resident CD103+CD8+ T cell response to acute viral infection remain unclear. Although TGFβ signaling is essential for CD103 expression, the precise molecular mechanism behind this regulation is elusive. Here, we reveal a TGFβ–SKI–Smad4 pathway that critically and specifically directs resident CD103+CD8+ T cell generation for protective immunity against primary and secondary viral infection. We found that resident CD103+CD8+ T cells are abundant in both lymphoid and nonlymphoid tissues from uninfected mice. CD103 acts as a costimulation signal to produce an optimal antigenic CD8+ T cell response to acute viral infection. There is a reduction in resident CD103+CD8+ T cells following primary infection that results in increased susceptibility of the host to secondary infection. Intriguingly, CD103 expression inversely and specifically correlates with SKI proto-oncogene (SKI) expression but not R-Smad2/3 activation. Ectopic expression of SKI restricts CD103 expression in CD8+ T cells in vitro and in vivo to hamper viral clearance. Mechanistically, SKI is recruited to the Itgae loci to directly suppress CD103 transcription by regulating histone acetylation in a Smad4-dependent manner. Our study therefore reveals that resident CD103+CD8+ T cells dictate protective immunity during primary and secondary infection. Interfering with SKI function may amplify the resident CD103+CD8+ T cell response to promote protective immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
ChIP-seq data are deposited in the GEO database under ID code GSE135533.
References
Rynda-Apple, A., Robinson, K. M. & Alcorn, J. F. Influenza and bacterial superinfection: illuminating the immunologic mechanisms of disease. Infect. Immun. 83, 3764–3770 (2015).
van der Sluijs, K. F. et al. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J. Immunol. 172, 7603–7609 (2004).
Brundage, J. F. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect. Dis. 6, 303–312 (2006).
Paget, C. & Trottein, F. Mechanisms of bacterial superinfection post-influenza: a role for unconventional T cells. Front. Immunol. 10, 336 (2019).
Ivanov, S. et al. Key role for respiratory CD103(+) dendritic cells, IFN-gamma, and IL-17 in protection against Streptococcus pneumoniae infection in response to alpha-galactosylceramide. J. Infect. Dis. 206, 723–734 (2012).
Wong, P. & Pamer, E. G. CD8 T cell responses to infectious pathogens. Annu. Rev. Immunol. 21, 29–70 (2003).
Restifo, N. P., Dudley, M. E. & Rosenberg, S. A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 12, 269–281 (2012).
Chang, J. T., Wherry, E. J. & Goldrath, A. W. Molecular regulation of effector and memory T cell differentiation. Nat. Immunol. 15, 1104–1115 (2014).
Gebhardt, T. et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10, 524–530 (2009).
Cepek, K. L. et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature 372, 190–193 (1994).
Iijima, N. & Iwasaki, A. Tissue instruction for migration and retention of TRM cells. Trends Immunol. 36, 556–564 (2015).
Hardenberg, J. B., Braun, A. & Schon, M. P. A Yin and Yang in epithelial immunology: the roles of the alphaE(CD103)beta7 Integrin in T Cells. J. investig. Dermatol. 138, 23–31 (2018).
Schlickum, S. et al. Integrin alpha E(CD103)beta 7 influences cellular shape and motility in a ligand-dependent fashion. Blood 112, 619–625 (2008).
Smazynski, J. & Webb, J. R. Resident memory-like tumor-infiltrating lymphocytes (TILRM): latest players in the immuno-oncology repertoire. Front. Immunol. 9, 1741 (2018).
Annacker, O. et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J. Exp. Med. 202, 1051–1061 (2005).
Duhen, T. et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 9, 2724 (2018).
Gabriely G. et al. Targeting latency-associated peptide promotes antitumor immunity. Sci. Immunol. 2, eaaj1738 (2017).
Shields, B. D. et al. Loss of E-cadherin inhibits CD103 antitumor activity and reduces checkpoint blockade responsiveness in melanoma. Cancer Res. 79, 1113–1123 (2019).
Boutet, M. et al. TGFbeta signaling intersects with CD103 integrin signaling to promote T-lymphocyte accumulation and antitumor activity in the lung tumor microenvironment. Cancer Res. 76, 1757–1769 (2016).
Abd Hamid, M. et al. Self-maintaining CD103(+) cancer-specific T cells are highly energetic with rapid cytotoxic and effector responses. Cancer Immunol. Res. 8, 203–216 (2020).
Franciszkiewicz, K. et al. CD103 or LFA-1 engagement at the immune synapse between cytotoxic T cells and tumor cells promotes maturation and regulates T-cell effector functions. Cancer Res. 73, 617–628 (2013).
Xue, D., Liu, P., Chen, W., Zhang, C. & Zhang, L. An anti-CD103 antibody-drug conjugate prolongs the survival of pancreatic islet allografts in mice. Cell Death Dis. 10, 735 (2019).
Hadley, G. A. & Higgins, J. M. Integrin alphaEbeta7: molecular features and functional significance in the immune system. Adv. Exp. Med. Biol. 819, 97–110 (2014).
Milner, J. J. & Goldrath, A. W. Transcriptional programming of tissue-resident memory CD8(+) T cells. Curr. Opin. Immunol. 51, 162–169 (2018).
Zhang, N. & Bevan, M. J. Transforming growth factor-beta signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity 39, 687–696 (2013).
El-Asady, R. et al. TGF-{beta}-dependent CD103 expression by CD8(+) T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J. Exp. Med. 201, 1647–1657 (2005).
Takimoto, T. et al. Smad2 and Smad3 are redundantly essential for the TGF-beta-mediated regulation of regulatory T plasticity and Th1 development. J. Immunol. 185, 842–855 (2010).
Li, M. O., Wan, Y. Y., Sanjabi, S., Robertson, A. K. & Flavell, R. A. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 24, 99–146 (2006).
Sun, Y., Liu, X., Ng-Eaton, E., Lodish, H. F. & Weinberg, R. A. SnoN and Ski protooncoproteins are rapidly degraded in response to transforming growth factor beta signaling. Proc. Natl Acad. Sci. USA 96, 12442–12447 (1999).
Deheuninck, J. & Luo, K. Ski and SnoN, potent negative regulators of TGF-beta signaling. Cell Res. 19, 47–57 (2009).
Bonthius, D. J. Lymphocytic choriomeningitis virus: an underrecognized cause of neurologic disease in the fetus, child, and adult. Semin Pediatr. Neurol. 19, 89–95 (2012).
Zhou, X., Ramachandran, S., Mann, M. & Popkin, D. L. Role of lymphocytic choriomeningitis virus (LCMV) in understanding viral immunology: past, present and future. Viruses 4, 2650–2669 (2012).
Grueter, B. et al. Runx3 regulates integrin alpha E/CD103 and CD4 expression during development of CD4-/CD8+ T cells. J. Immunol. 175, 1694–1705 (2005).
Andrew, D. P., Rott, L. S., Kilshaw, P. J. & Butcher, E. C. Distribution of alpha 4 beta 7 and alpha E beta 7 integrins on thymocytes, intestinal epithelial lymphocytes and peripheral lymphocytes. Eur. J. Immunol. 26, 897–905 (1996).
Fousteri, G. et al. Minimal effect of CD103 expression on the control of a chronic antiviral immune response. Viral Immunol. 23, 285–294 (2010).
Dutko, F. J. & Oldstone, M. B. Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J. Gen. Virol. 64(Pt 8), 1689–1698 (1983).
Murali-Krishna, K. et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8, 177–187 (1998).
Mackay, L. K. et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 14, 1294–1301 (2013).
Chytil, A., Magnuson, M. A., Wright, C. V. & Moses, H. L. Conditional inactivation of the TGF-beta type II receptor using Cre:Lox. Genesis 32, 73–75 (2002).
Lee, P. P. et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15, 763–774 (2001).
Akiyoshi, S. et al. c-Ski acts as a transcriptional co-repressor in transforming growth factor-beta signaling through interaction with smads. J. Biol. Chem. 274, 35269–35277 (1999).
Li, M. O., Sanjabi, S. & Flavell, R. A. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25, 455–471 (2006).
Marie, J. C., Liggitt, D. & Rudensky, A. Y. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity 25, 441–454 (2006).
Liu, Y. et al. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat. Immunol. 9, 632–640 (2008).
Gu, A. D., Wang, Y., Lin, L., Zhang, S. S. & Wan, Y. Y. Requirements of transcription factor Smad-dependent and -independent TGF-beta signaling to control discrete T-cell functions. Proc. Natl Acad. Sci. USA 109, 905–910 (2012).
Zhang, S. et al. Reversing SKI-SMAD4-mediated suppression is essential for TH17 cell differentiation. Nature 551, 105–109 (2017).
Chu, G. C., Dunn, N. R., Anderson, D. C., Oxburgh, L. & Robertson, E. J. Differential requirements for Smad4 in TGFbeta-dependent patterning of the early mouse embryo. Development 131, 3501–3512 (2004).
Wang, Z. et al. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell 138, 1019–1031 (2009).
Amsen, D., van Gisbergen, K., Hombrink, P. & van Lier, R. A. W. Tissue-resident memory T cells at the center of immunity to solid tumors. Nat. Immunol. 19, 538–546 (2018).
Park, C. O. & Kupper, T. S. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat. Med. 21, 688–697 (2015).
Woon, H. G. et al. Compartmentalization of total and virus-specific tissue-resident memory CD8+ T cells in human lymphoid organs. PLoS Pathog. 12, e1005799 (2016).
Sathaliyawala, T. et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 38, 187–197 (2013).
Laidlaw, B. J. et al. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity 41, 633–645 (2014).
Mackay, L. K. et al. T-box transcription factors combine with the cytokines TGF-beta and IL-15 to control tissue-resident memory T cell fate. Immunity 43, 1101–1111 (2015).
Pearce, E. L. et al. Control of effector CD8+ T cell function by the transcription factor eomesodermin. Science 302, 1041–1043 (2003).
Sullivan, B. M., Juedes, A., Szabo, S. J., von Herrath, M. & Glimcher, L. H. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc. Natl Acad. Sci. USA 100, 15818–15823 (2003).
Kaech, S. M. & Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12, 749–761 (2012).
Mokrani, M., Klibi, J., Bluteau, D., Bismuth, G. & Mami-Chouaib, F. Smad and NFAT pathways cooperate to induce CD103 expression in human CD8 T lymphocytes. J. Immunol. 192, 2471–2479 (2014).
Tu, E. et al. T cell receptor-regulated TGF-beta type I receptor expression determines T cell quiescence and activation. Immunity 48, e746 (2018).
Nagano, Y. et al. Arkadia induces degradation of SnoN and c-Ski to enhance transforming growth factor-beta signaling. J. Biol. Chem. 282, 20492–20501 (2007).
Tecalco-Cruz, A. C., Rios-Lopez, D. G., Vazquez-Victorio, G., Rosales-Alvarez, R. E. & Macias-Silva, M. Transcriptional cofactors Ski and SnoN are major regulators of the TGF-beta/Smad signaling pathway in health and disease. Signal Transduct. Target. Ther. 3, 15 (2018).
Nomura, T. et al. Ski is a component of the histone deacetylase complex required for transcriptional repression by Mad and thyroid hormone receptor. Genes Dev. 13, 412–423 (1999).
Tabata, T., Kokura, K., Ten Dijke, P. & Ishii, S. Ski co-repressor complexes maintain the basal repressed state of the TGF-beta target gene, SMAD7, via HDAC3 and PRMT5. Genes Cells 14, 17–28 (2009).
Acknowledgements
We thank E. Robertson and E. Bikoff for providing Smad4fl/fl mice, H. Moses for providing TgfbrIIfl/fl mice, and the NIH tetramer core facility at Emory University for providing the tetramers. This work was supported by NIH funding (R01AI143894; R01AI138337) for J.K.W.; the NIH (AI123193); the National Multiple Sclerosis Society (RG-1802-30483); and the Yang Family Biomedical Scholars Award for Y.Y.W.
Author information
Authors and Affiliations
Contributions
B.W. and G.Z. contributed equally to the design and implementation of the cellular, molecular, biochemical, and animal experiments; B.W. contributed to the writing of the manuscript; Z.G., G.W., and J. Z. contributed to the generation and characterization of SKI-KI mice; J.L. and X.X. contributed to the bioinformatic analysis of ChIP data; J.K.W. contributed to the LCMV infection model; and Y.Y.W. conceived the project, designed the experiments, and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Wu, B., Zhang, G., Guo, Z. et al. The SKI proto-oncogene restrains the resident CD103+CD8+ T cell response in viral clearance. Cell Mol Immunol 18, 2410–2421 (2021). https://doi.org/10.1038/s41423-020-0495-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41423-020-0495-7
Keywords
This article is cited by
-
c-Ski is a novel repressor of NF-κB through interaction with p65 and HDAC1 in U937 cells
Cell Communication and Signaling (2025)
-
TGFβ control of immune responses in cancer: a holistic immuno-oncology perspective
Nature Reviews Immunology (2023)
-
Immunological characteristics of CD103+CD8+ Tc cells in the liver of C57BL/6 mouse infected with plasmodium NSM
Parasitology Research (2023)