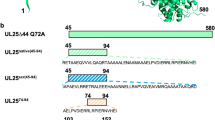
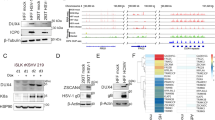
Abstract
Inflammasomes play important roles in resisting infections caused by various pathogens. HSV-1 is a highly contagious virus among humans. The process by which HSV-1 particles bud from the nucleus is unique to herpes viruses, but the specific mechanism is still unclear. Here, we screened genes involved in HSV-1 replication. We found that TET3 plays an essential role in HSV-1 infection. TET3 recognizes the UL proteins of HSV-1 and, upon activation, can directly bind to caspase-1 to activate an ASC-independent inflammasome in the nucleus. The subsequent cleavage of GSDMD in the nucleus is crucial for the budding of HSV-1 particles from the nucleus. Inhibiting the perforation ability of GSDMD on the nuclear membrane can significantly reduce the maturation and spread of HSV-1. Our results may provide a new approach for the treatment of HSV-1 in the future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Fu J, Wu H. Structural Mechanisms of NLRP3 Inflammasome Assembly and Activation. Annu Rev Immunol. 2023;41:301–16.
Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, et al. Caspase-11 cleaves gasdermin D for noncanonical inflammasome signaling. Nature. 2015;526:666–71.
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–5.
Zhu F, Ma J, Li W, Liu Q, Qin X, Qian Y, et al. The orphan receptor Nur77 binds cytoplasmic LPS to activate the noncanonical NLRP3 inflammasome. Immunity. 2023;56:753–67.e758.
Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477:592–5.
Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–9.
Megli CJ, Coyne CB. Infections at the maternal-fetal interface: an overview of pathogenesis and defense. Nat Rev Microbiol. 2022;20:67–82.
Walker FC, Sridhar PR, Baldridge MT. Differential roles of interferons in innate responses to mucosal viral infections. Trends Immunol. 2021;42:1009–23.
Johnson KE, Chikoti L, Chandran B. Herpes simplex virus 1 infection induces activation and subsequent inhibition of the IFI16 and the NLRP3 inflammasome. J Virol. 2013;87:5005–18.
Zhang M, Covar J, Zhang NY, Chen W, Marshall B, Mo J, et al. Virus spread and immune response following anterior chamber inoculation of HSV-1 lacking the Beclin-binding domain (BBD). J Neuroimmunol. 2013;260:82–91.
Bigalke JM, Heuser T, Nicastro D, Heldwein EE. Membrane deformation and scission by the HSV-1 nuclear egress complex. Nat Commun. 2014;5:4131.
Johnson DC, Baines JD. Herpesviruses remodel host membranes for virus egress. Nat Rev Microbiol. 2011;9:382–94.
Ravindran MS, Bagchi P, Cunningham CN, Tsai B. Opportunistic intruders: how viruses orchestrate ER functions to infect cells. Nat Rev Microbiol. 2016;14:407–20.
Romero-Brey I, Bartenschlager R. Endoplasmic Reticulum: The Favorite Intracellular Niche for Viral Replication and Assembly. Viruses. 2016;8:160.
Cavalli G, Heard E. Advances in epigenetics link genetics to the environment and disease. Nature. 2019;571:489–99.
Lo YMD, Han DSC, Jiang P, Chiu RWK. Epigenetics, fragmentomics, and topology of cell-free DNA in liquid biopsies. Science 2021;372:eaaw3616.
Gu TP, Guo F, Yang H, Wu HP, Xu GF, Liu W, et al. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature. 2011;477:606–10.
He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q, et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333:1303–7.
Shen Q, Zhang Q, Shi Y, Shi Q, Jiang Y, Gu Y, et al. Tet2 promotes pathogen infection-induced myelopoiesis through mRNA oxidation. Nature. 2018;554:123–7.
Xue S, Liu C, Sun X, Li W, Zhang C, Zhou X, et al. TET3 Inhibits Type I IFN Production Independent of DNA Demethylation. Cell Rep. 2016;16:1096–105.
Zhang Q, Zhao K, Shen Q, Han Y, Gu Y, Li X, et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature. 2015;525:389–93.
Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–73.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
Kim A, Choi SJ, Song GG, Kim JH, Jung JH. Characterization of virus-mediated autoimmunity and the consequences for pathological process in patients with systemic lupus erythematosus. Clin Rheumatol. 2023;42:2799–809.
van Gent M, Chiang JJ, Muppala S, Chiang C, Azab W, Kattenhorn L, et al. The US3 Kinase of Herpes Simplex Virus Phosphorylates the RNA Sensor RIG-I To Suppress Innate Immunity. J Virol. 2022;96:e0151021.
Wang W, Hu D, Wu C, Feng Y, Li A, Liu W, et al. STING promotes NLRP3 localization in ER and facilitates NLRP3 deubiquitination to activate the inflammasome upon HSV-1 infection. PLoS Pathog. 2020;16:e1008335.
Mohnke J, Stark I, Fischer M, Fischer PM, Schlosser A, Grothey A, et al. pUL36 Deubiquitinase Activity Augments Both the Initiation and the Progression of Lytic Herpes Simplex Virus Infection in IFN-Primed Cells. J Virol. 2022;96:e0096322.
Packard JE, Williams MR, Fromuth DP, Dembowski JA. Proliferating cell nuclear antigen inhibitors block distinct stages of herpes simplex virus infection. PLoS Pathog. 2023;19:e1011539.
Klupp BG, Mettenleiter TC. The Knowns and Unknowns of Herpesvirus Nuclear Egress. Annu Rev Virol. 2023;10:305–23.
Calbay O, Padia R, Akter M, Sun L, Li B, Qian N, et al. ASC/inflammasome-independent pyroptosis in ovarian cancer cells through translational augmentation of caspase-1. iScience. 2023;26:108408.
Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao E. A Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–3.
Fu R, Zhao L, Guo Y, Qin X, Xu W, Cheng X et al. AIM2 inflammasome: A potential therapeutic target in ischemic stroke. Clin Immunol. 2024;259:109881.
Devant P, Kagan JC. Molecular mechanisms of gasdermin D pore-forming activity. Nat Immunol. 2023;24:1064–75.
Martin BN, Wang C, Willette-Brown J, Herjan T, Gulen MF, Zhou H, et al. IKKα negatively regulates ASC-dependent inflammasome activation. Nat Commun. 2014;5:4977.
Smatlik N, Drexler S, K, Burian M, Röcken M, Yazdi AS. ASC Speck Formation after Inflammasome Activation in Primary Human Keratinocytes. Oxid Med Cell Longev. 2021;2021:7914829.
Peng X, Na R, Zhou W, Meng X, Yang Y, Amini S, et al. Nuclear translocation of Gasdermin D sensitizes colorectal cancer to chemotherapy in a pyroptosis-independent manner. Oncogene. 2022;41:5092–106.
Wang L, Fu H, Nanayakkara G, Li Y, Shao Y, Johnson C, et al. Novel extracellular and nuclear caspase-1 and inflammasomes propagate inflammation and regulate gene expression: a comprehensive database mining study. J Hematol Oncol. 2016;9:122.
Xia S, Zhang Z, Magupalli VG, Pablo JL, Dong Y, Vora SM, et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature. 2021;593:607–11.
Miao R, Jiang C, Chang WY, Zhang H, An J, Ho F, et al. Gasdermin D permeabilization of mitochondrial inner and outer membranes accelerates and enhances pyroptosis. Immunity. 2023;56:2523–41.e2528.
Orzalli MH, Prochera A, Payne L, Smith A, Garlick JA, Kagan JC. Virus-mediated inactivation of anti-apoptotic Bcl-2 family members promotes Gasdermin-E-dependent pyroptosis in barrier epithelial cells. Immunity. 2021;54:1447–62.e1445.
Graham JM Isolation of nuclei and nuclear membranes from animal tissues. Curr Protoc Cell Biol. 2001;Chapter 3:3.10.11-13.10.19.
Acknowledgements
We thank Yingyu Chen (Peking University) for technical help. This work was supported by the National Natural Science Foundation of China (92369104, 82271790, 92169113), the Beijing Natural Science Foundation (JQ23028, 7212067), the National Key R&D Program of China (2021YFA1300202, 2022YFC2302900), the Strategic Priority Research Programs of the Chinese Academy of Sciences (XDB29020000), the Key Research Program of Frontier Sciences of Chinese Academy of Sciences (ZDBS-LY-SM025), the CAS Project for Young Scientists in Basic Research (YSBR-010), the Fok Ying Tung Education Foundation to P.X., the Youth Innovation Promotion Association of CAS to S.W.
Author information
Authors and Affiliations
Contributions
Q.L. and W.L. performed the experiments and analyzed the data; Y.Q., C.W., C.K., M.L., LL.S. and L.S. performed the experiments; S.W., Y.P. and C.J. analyzed the data; P.X. initiated the study, designed and performed the experiments, analyzed the data, and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Q., Li, W., Qian, Y. et al. The TET3 inflammasome senses unique long HSV-1 proteins for virus particle budding from the nucleus. Cell Mol Immunol 21, 1322–1334 (2024). https://doi.org/10.1038/s41423-024-01221-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41423-024-01221-2
Keywords
This article is cited by
-
Eukaryotic ADCY7 catalyzes the production of c-di-AMP to activate the NLRP3 inflammasome
Nature Chemical Biology (2025)