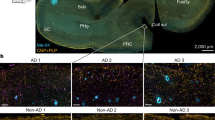
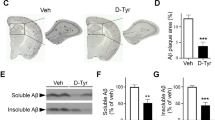
Abstract
Microglia dysfunction-associated neuroinflammation is an important driver of Alzheimer’s disease (AD), but the mechanism is poorly understood. Here, we show that demyelination promotes neuroinflammation and cognitive impairment via the lysophosphatidylserine (LysoPS)-GPR34 axis in AD. Demyelination is observed at the early stage and is accompanied by an increase in LysoPS in myelin debris in a 5xFAD mouse model of AD. Reducing the content of LysoPS in myelin or inhibiting its receptor GPR34 via genetic or pharmacological approaches can reduce microglial dysfunction and neuroinflammation and improve microglial Aβ phagocytosis, subsequently resulting in less Aβ deposition and memory restoration in 5xFAD mice. Furthermore, increased LysoPS production and microglial GPR34 expression were also observed in the brains of AD patients. These results reveal the pathogenic role of demyelination-derived LysoPS in microglial dysfunction and AD pathology and suggest that blocking GPR34 as a therapeutic strategy beyond targeting Aβ.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The sequencing data reported in this article have been deposited in the Gene Expression Omnibus (GEO accession number: GSE216209). Additional data supporting the presented findings are available in the manuscript and upon request from the corresponding author. All data are available in the main text or the supplementary materials. All the cell lines generated in this study are available from the authors.
References
Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6.
Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–5.
Karran E, De Strooper B. The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat Rev Drug Discov. 2022;21:306–18.
Small SA, Duff K. Linking Abeta and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron. 2008;60:534–42.
Herrup K. The case for rejecting the amyloid cascade hypothesis. Nat Neurosci. 2015;18:794–9.
Reardon S. Alzheimer’s drug donanemab: what promising trial means for treatments. Nature. 2023;617:232–3.
Travis J. Latest Alzheimer’s antibody is ‘not a miracle drug’. Science. 2023;380:571.
Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol. 2021;17:157–72.
Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–8.
Tarkowski E, Andreasen N, Tarkowski A, Blennow K. Intrathecal inflammation precedes development of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:1200–5.
Wright AL, Zinn R, Hohensinn B, Konen LM, Beynon SB, Tan RP, et al. Neuroinflammation and neuronal loss precede Abeta plaque deposition in the hAPP-J20 mouse model of Alzheimer’s disease. PLoS One. 2013;8:e59586.
Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–51.
Okello A, Edison P, Archer HA, Turkheimer FE, Kennedy J, Bullock R, et al. Microglial activation and amyloid deposition in mild cognitive impairment: a PET study. Neurology. 2009;72:56–62.
Femminella GD, Dani M, Wood M, Fan Z, Calsolaro V, Atkinson R, et al. Microglial activation in early Alzheimer trajectory is associated with higher gray matter volume. Neurology. 2019;92:e1331–e1343.
Kreisl WC, Lyoo CH, McGwier M, Snow J, Jenko KJ, Kimura N, et al. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain. 2013;136:2228–38.
Möller HJ, Graeber MB. The case described by Alois Alzheimer in 1911. Historical and conceptual perspectives based on the clinical record and neurohistological sections. Eur Arch Psychiatry Clin Neurosci. 1998;248:111–22.
Couttas TA, Kain N, Suchowerska AK, Quek LE, Turner N, Fath T, et al. Loss of ceramide synthase 2 activity, necessary for myelin biosynthesis, precedes tau pathology in the cortical pathogenesis of Alzheimer’s disease. Neurobiol Aging. 2016;43:89–100.
Desai MK, Sudol KL, Janelsins MC, Mastrangelo MA, Frazer ME, Bowers WJ. Triple-transgenic Alzheimer’s disease mice exhibit region-specific abnormalities in brain myelination patterns prior to appearance of amyloid and tau pathology. Glia. 2009;57:54–65.
Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science. 2005;307:1282–8.
Zhang X, Wang R, Hu D, Sun X, Fujioka H, Lundberg K, et al. Oligodendroglial glycolytic stress triggers inflammasome activation and neuropathology in Alzheimer’s disease. Sci Adv. 2020;6:eabb8680.
Depp, C, et al. Myelin dysfunction drives amyloid-beta deposition in models of Alzheimer’s disease. Nature. 2023;618:349–357.
Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl J Med. 2013;368:107–16.
Damisah EC, Rai A, Grutzendler J. TREM2: modulator of lipid metabolism in microglia. Neuron. 2020;105:759–61.
Wood H. TREM2 activation promotes remyelination. Nat Rev Neurol. 2020;16:522–522.
Li R-Y, Qin Q, Yang HC, Wang YY, Mi YX, Yin YS, et al. TREM2 in the pathogenesis of AD: a lipid metabolism regulator and potential metabolic therapeutic target. Mol Neurodegeneration. 2022;17:40.
Williams K, Ulvestad E, Waage A, Antel JP, McLaurin J. Activation of adult human derived microglia by myelin phagocytosis in vitro. J Neurosci Res. 1994;38:433–43.
Cantuti-Castelvetri L, Fitzner D, Bosch-Queralt M, Weil MT, Su M, Sen P, et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science. 2018;359:684–8.
Lin B, Zhou Y, Huang Z, Ma M, Qi M, Jiang Z, et al. GPR34 senses demyelination to promote neuroinflammation and pathologies. Cell Mol Immunol. 2024;21:1131–1144.
McLaurin J, Franklin T, Chakrabartty A, Fraser PE. Phosphatidylinositol and inositol involvement in Alzheimer amyloid-β fibril growth and arrest. J Mol Biol. 1998;278:183–94.
Ando K, Erneux C, Homa M, Houben S, de Fisenne MA, Brion JP, et al. Dysregulation of phosphoinositide 5-phosphatases and phosphoinositides in Alzheimer’s disease. Front Neurosci. 2021;15:614855.
Ma X, Li X, Wang W, Zhang M, Yang B, Miao Z. Phosphatidylserine, inflammation, and central nervous system diseases. Front Aging Neurosci. 2022;14:975176.
Kamat SS, Camara K, Parsons WH, Chen DH, Dix MM, Bird TD, et al. Immunomodulatory lysophosphatidylserines are regulated by ABHD16A and ABHD12 interplay. Nat Chem Biol. 2015;11:164–71.
Aoki J, Nagai Y, Hosono H, Inoue K, Arai H. Structure and function of phosphatidylserine-specific phospholipase A1. Biochim Biophys Acta. 2002;1582:26–32.
Shanbhag K, Mhetre A, Khandelwal N, Kamat SS. The lysophosphatidylserines—an emerging class of signaling lysophospholipids. J Membr Biol. 2020;253:381–97.
Heckmann BL, Teubner BJW, Tummers B, Boada-Romero E, Harris L, Yang M, et al. LC3-associated endocytosis facilitates β-amyloid clearance and mitigates neurodegeneration in murine Alzheimer’s disease. Cell. 2019;178:536–551.e14.
Ennerfelt H, Frost EL, Shapiro DA, Holliday C, Zengeler KE, Voithofer G, et al. SYK coordinates neuroprotective microglial responses in neurodegenerative disease. Cell. 2022;185:4135–4152.
Kandalepas PC, Sadleir KR, Eimer WA, Zhao J, Nicholson DA, Vassar R. The Alzheimer’s beta-secretase BACE1 localizes to normal presynaptic terminals and to dystrophic presynaptic terminals surrounding amyloid plaques. Acta Neuropathol. 2013;126:329–52.
Roy ER, Chiu G, Li S, Propson NE, Kanchi R, Wang B, et al. Concerted type I interferon signaling in microglia and neural cells promotes memory impairment associated with amyloid β plaques. Immunity. 2022;55:879–894.e6.
Clarner T, Diederichs F, Berger K, Denecke B, Gan L, van der Valk P, et al. Myelin debris regulates inflammatory responses in an experimental demyelination animal model and multiple sclerosis lesions. Glia. 2012;60:1468–80.
Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J neuroinflammation. 2015;12:1–13.
Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 2017;47:566–581.e9.
Chen XY, Feng SN, Bao Y, Zhou YX, Ba F. Identification of Clec7a as the therapeutic target of rTMS in alleviating Parkinson’s disease: targeting neuroinflammation. Biochim Biophys Acta Mol Basis Dis. 2023;1869:166814.
Omi J, Kano K, Aoki J. Current knowledge on the biology of lysophosphatidylserine as an emerging bioactive lipid. Cell Biochem Biophys. 2021;79:497–508.
Inoue A, Ishiguro J, Kitamura H, Arima N, Okutani M, Shuto A, et al. TGFα shedding assay: an accurate and versatile method for detecting GPCR activation. Nat methods. 2012;9:1021–9.
Yao Z, van Velthoven C, Kunst M, Zhang M, McMillen D, Lee C, et al. A high-resolution transcriptomic and spatial atlas of cell types in the whole mouse brain. Nature. 2023;624:317–32.
Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, et al. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci. 2013;16:1896–905.
Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17:131–43.
Yu Y, Ye RD. Microglial Aβ receptors in Alzheimer’s disease. Cell Mol Neurobiol. 2015;35:71–83.
De Roeck A, Van Broeckhoven C, Sleegers K. The role of ABCA7 in Alzheimer’s disease: evidence from genomics, transcriptomics and methylomics. Acta Neuropathologica. 2019;138:201–20.
Iribarren P, Zhou Y, Hu J, Le Y, Wang JM. Role of formyl peptide receptor-like 1 (FPRL1/FPR2) in mononuclear phagocyte responses in Alzheimer disease. Immunologic Res. 2005;31:165–76.
Lin L-L, Song GJ, Zhang H, Yin Y, Xin SM, Ding L, et al. GPR34 knockdown relieves cognitive deficits and suppresses neuroinflammation in Alzheimer’s disease via the ERK/NF-κB signal. Neuroscience. 2023;528:129–39.
Shi Y, Andhey PS, Ising C, Wang K, Snipes LL, Boyer K, et al. Overexpressing low-density lipoprotein receptor reduces tau-associated neurodegeneration in relation to apoE-linked mechanisms. Neuron. 2021;109:2413–2426.e7.
Hill RA, Li AM, Grutzendler J. Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat Neurosci. 2018;21:683–95.
Sayo A, Konishi H, Kobayashi M, Kano K, Kobayashi H, Hibi H, et al. GPR34 in spinal microglia exacerbates neuropathic pain in mice. J Neuroinflammation. 2019;16:1–11.
Chen J-F, Liu K, Hu B, Li RR, Xin W, Chen H, et al. Enhancing myelin renewal reverses cognitive dysfunction in a murine model of Alzheimer’s disease. Neuron. 2021;109:2292–-2307.e5.
Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–72.
Ising C, Venegas C, Zhang S, Scheiblich H, Schmidt SV, Vieira-Saecker A, et al. NLRP3 inflammasome activation drives tau pathology. Nature. 2019;575:669–73.
Labzin LI, Heneka MT, Latz E. Innate Immunity and Neurodegeneration. Annu Rev Med. 2018;69:437–49.
Bédard A, Tremblay P, Chernomoretz A, Vallières L. Identification of genes preferentially expressed by microglia and upregulated during cuprizone-induced inflammation. Glia. 2007;55:777–89.
Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, et al. Aging as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15:565–81.
Safaiyan S, Kannaiyan N, Snaidero N, Brioschi S, Biber K, Yona S, et al. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci. 2016;19:995–8.
Makide K, Aoki J. GPR34 as a lysophosphatidylserine receptor. J Biochem. 2013;153:327–9.
Izume, T, et al. Structural basis for lysophosphatidylserine recognition by GPR34. bioRxiv, 2023: 2023.02. 15.528751.
Aisen PS. The potential of anti-inflammatory drugs for the treatment of Alzheimer’s disease. Lancet Neurol. 2002;1:279–84.
Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289:2819–26.
Zhao Y, Wu X, Li X, Jiang LL, Gui X, Liu Y, et al. TREM2 is a receptor for beta-amyloid that mediates microglial function. Neuron. 2018;97:1023–1031.e7.
Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, et al. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78:631–43.
Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–65.
Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–74.
Wang X, Cai J, Lin B, Ma M, Tao Y, Zhou Y, et al. GPR34-mediated sensing of lysophosphatidylserine released by apoptotic neutrophils activates type 3 innate lymphoid cells to mediate tissue repair. Immunity. 2021;54:1123–1136.e8.
Sun X, Wang X, Chen T, Li T, Cao K, Lu A, et al. Myelin activates FAK/Akt/NF-kappaB pathways and provokes CR3-dependent inflammatory response in murine system. PLoS One. 2010;5:e9380.
Stine, WB, et al., Preparing synthetic Aβ in different aggregation states, in Alzheimer’s Disease and Frontotemporal Dementia. 2010, Springer. 13-32
Brewer GJ, Torricelli JR. Isolation and culture of adult neurons and neurospheres. Nat Protoc. 2007;2:1490–8.
Chiang ACA, Seua AV, Singhmar P, Arroyo LD, Mahalingam R, Hu J, et al. Bexarotene normalizes chemotherapy-induced myelin decompaction and reverses cognitive and sensorimotor deficits in mice. Acta Neuropathol Commun. 2020;8:193.
Xia A, Yong X, Zhang C, Lin G, Jia G, Zhao C, et al. Cryo-EM structures of human GPR34 enable the identification of selective antagonists. Proc Natl Acad Sci. 2023;120:e2308435120.
Cammarota M, Boscia F. Contribution of oligodendrocytes, microglia, and astrocytes to myelin debris uptake in an explant model of inflammatory demyelination in rats. Cells. 2023;12:2203.
Acknowledgements
We thank Dr. Hui Fu for providing the Cnp-Cre mice. Human brain samples were provided by the National Health and Disease Human Brain Tissue Resource Center (Anhui Medical University). This research was supported by the National Key Research and Development Program of China (grant number 2020YFA0509101), the National Natural Science Foundation of China (grant numbers 81821001, 82130107, U20A20359), and the CAS Project for Young Scientists in Basic Research (YSBR-074).
Author information
Authors and Affiliations
Contributions
YZ, XW, ZH, BL, YH, JL WX and MM performed the experiments; YZ, GH, WM, XW, WJ and RZ designed the research. YZ, XW, WJ and RZ wrote the manuscript. WJ and RZ supervised the project.
Corresponding authors
Ethics declarations
Competing interests
BL, WJ and RZ are co-inventors of a pending patent application (202110355421.6) submitted by University of Science and Technology of China. All the other authors declare that they have no competing interests. RZ is Deputy Editor-in-Chief of Cellular & Molecular Immunology, but he has not been involved in the peer review or the decision-making of the article.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Y., Huang, Z., Lin, B. et al. Demyelination-derived lysophosphatidylserine promotes microglial dysfunction and neuropathology in a mouse model of Alzheimer’s disease. Cell Mol Immunol 22, 134–149 (2025). https://doi.org/10.1038/s41423-024-01235-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41423-024-01235-w
Keywords
This article is cited by
-
Emerging connections between myelin and Alzheimer’s disease
Nature Cell Biology (2025)
-
Myelin debris as an initiator of microglial dysfunction and neuropathology in Alzheimer’s disease
Cellular & Molecular Immunology (2025)