Abstract
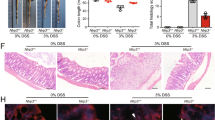
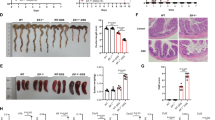
Group 3 innate lymphoid cells (ILC3s) control tissue homeostasis and orchestrate mucosal inflammation; however, the precise mechanisms governing ILC3 activity are fully understood. Here, we identified the transmembrane protein neuropilin-1 (NRP1) as a positive regulator of interleukin (IL)-17-producing ILC3s in the intestine. NRP1 was markedly upregulated in intestinal mucosal biopsies from patients with inflammatory bowel disease (IBD) compared with healthy controls. Genetic deficiency of NRP1 reduces the frequency of ILC3s in the gut and impairs their production of IL-17A in an NF-κB signaling-dependent and cell-intrinsic manner. The diminished IL-17A production in ILC3s altered the composition of the microbiota and improved the outcome of dextran sodium sulfate (DSS)-induced colitis. Furthermore, pharmacological inhibition of NRP1 with EG00229 alleviated the severity of colitis. These observations demonstrated the critical role of NRP1 in the control of intestinal ILC3s, suggesting that NRP1 is a potential therapeutic target for IBD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–7.
Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42.
Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621.
Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205–17.
Danese S, Fiocchi C. Ulcerative colitis. N. Engl J Med. 2011;365:1713–25.
Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–605.
Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–42.
Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–17.
Vereecke L, Beyaert R, van Loo G. Enterocyte death and intestinal barrier maintenance in homeostasis and disease. Trends Mol Med. 2011;17:584–93.
Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288.
Kim HY, Lee HJ, Chang YJ, Pichavant M, Shore SA, Fitzgerald KA, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. 2014;20:54–61.
Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells-a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–9.
Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306.
Lyu M, Suzuki H, Kang L, Gaspal F, Zhou W, Goc J, et al. ILC3s select microbiota-specific regulatory T cells to establish tolerance in the gut. Nature. 2022;610:744–51.
Kedmi R, Najar TA, Mesa KR, Grayson A, Kroehling L, Hao Y, et al. A RORgammat(+) cell instructs gut microbiota-specific T(reg) cell differentiation. Nature. 2022;610:737–43.
Li J, Doty AL, Tang Y, Berrie D, Iqbal A, Tan SA, et al. Enrichment of IL-17A(+) IFN-gamma(+) and IL-22(+) IFN-gamma(+) T-cell subsets is associated with reduction of NKp44(+) ILC3s in the terminal ileum of Crohn’s disease patients. Clin Exp Immunol. 2017;190:143–53.
Martin JC, Chang C, Boschetti G, Ungaro R, Giri M, Grout JA, et al. Single-Cell Analysis of Crohn’s Disease Lesions Identifies a Pathogenic Cellular Module Associated with Resistance to Anti-TNF Therapy. Cell. 2019;178:1493–1508 e1420.
Hepworth MR, Fung TC, Masur SH, Kelsen JR, McConnell FM, Dubrot J, et al. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4(+) T cells. Science. 2015;348:1031–5.
Zhou L, Chu C, Teng F, Bessman NJ, Goc J, Santosa EK, et al. Innate lymphoid cells support regulatory T cells in the intestine through interleukin-2. Nature. 2019;568:405–9.
Zhou L, Zhou W, Joseph AM, Chu C, Putzel GG, Fang B, et al. Group 3 innate lymphoid cells produce the growth factor HB-EGF to protect the intestine from TNF-mediated inflammation. Nat Immunol. 2022;23:251–61.
Goldberg R, Prescott N, Lord GM, MacDonald TT, Powell N. The unusual suspects-innate lymphoid cells as novel therapeutic targets in IBD. Nat Rev Gastroenterol Hepatol. 2015;12:271–83.
He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–51.
Takagi S, Kasuya Y, Shimizu M, Matsuura T, Tsuboi M, Kawakami A, et al. Expression of a cell adhesion molecule, neuropilin, in the developing chick nervous system. Dev Biol. 1995;170:207–22.
Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–62.
Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–45.
Bruder D, Probst-Kepper M, Westendorf AM, Geffers R, Beissert S, Loser K, et al. Neuropilin-1: a surface marker of regulatory T cells. Eur J Immunol. 2004;34:623–30.
Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013;501:252–6.
Leclerc M, Voilin E, Gros G, Corgnac S, de Montpréville V, Validire P, et al. Regulation of antitumour CD8 T-cell immunity and checkpoint blockade immunotherapy by Neuropilin-1. Nat Commun. 2019;10:3345.
Liu C, Somasundaram A, Manne S, Gocher AM, Szymczak-Workman AL, Vignali KM, et al. Neuropilin-1 is a T-cell memory checkpoint limiting long-term antitumor immunity. Nat Immunol. 2020;21:1010–21.
Zhang J, Qiu J, Zhou W, Cao J, Hu X, Mi W, et al. Neuropilin-1 mediates lung tissue-specific control of ILC2 function in type 2 immunity. Nat Immunol. 2022;23:237–50.
Zheng, X. et al. Neuropilin-1(high) monocytes protect against neonatal inflammation. Cell Mol Immunol. 2024;21:575–88.
Haberman Y, Karns R, Dexheimer PJ, Schirmer M, Somekh J, Jurickova I, et al. Ulcerative colitis mucosal transcriptomes reveal mitochondriopathy and personalized mechanisms underlying disease severity and treatment response. Nat Commun. 2019;10:38.
Haberman Y, Tickle TL, Dexheimer PJ, Kim MO, Tang D, Karns R, et al. Pediatric Crohn disease patients exhibit specific ileal transcriptome and microbiome signature. J Clin Invest. 2014;124:3617–33.
Gury-BenAri M, Thaiss CA, Serafini N, Winter DR, Giladi A, Lara-Astiaso D, et al. The Spectrum and Regulatory Landscape of Intestinal Innate Lymphoid Cells Are Shaped by the Microbiome. Cell. 2016;166:1231–46.e1213.
Hoorweg K, Peters CP, Cornelissen F, Aparicio-Domingo P, Papazian N, Kazemier G, et al. Functional Differences between Human NKp44(-) and NKp44(+) RORC(+) Innate Lymphoid Cells. Front Immunol. 2012;3:72.
Björklund ÅK, Forkel M, Picelli S, Konya V, Theorell J, Friberg D, et al. The heterogeneity of human CD127(+) innate lymphoid cells revealed by single-cell RNA sequencing. Nat Immunol. 2016;17:451–60.
Fu T, Li Y, Oh TG, Cayabyab F, He N, Tang Q, et al. FXR mediates ILC-intrinsic responses to intestinal inflammation. Proc Natl Acad Sci USA. 2022;119:2213041119.
Linley H, Ogden A, Jaigirdar S, Buckingham L, Cox J, Priestley M, et al. CD200R1 promotes interleukin-17 production by group 3 innate lymphoid cells by enhancing signal transducer and activator of transcription 3 activation. Mucosal Immunol. 2023;16:167–79.
Bielecki P, Riesenfeld SJ, Hütter JC, Torlai Triglia E, Kowalczyk MS, Ricardo-Gonzalez RR, et al. Skin-resident innate lymphoid cells converge on a pathogenic effector state. Nature. 2021;592:128–32.
Mao K, Baptista AP, Tamoutounour S, Zhuang L, Bouladoux N, Martins AJ, et al. Innate and adaptive lymphocytes sequentially shape the gut microbiota and lipid metabolism. Nature. 2018;554:255–9.
Alenghat T, Osborne LC, Saenz SA, Kobuley D, Ziegler CG, Mullican SE, et al. Histone deacetylase 3 coordinates commensal-bacteria-dependent intestinal homeostasis. Nature. 2013;504:153–7.
Bessman NJ, Mathieu J, Renassia C, Zhou L, Fung TC, Fernandez KC, et al. Dendritic cell-derived hepcidin sequesters iron from the microbiota to promote mucosal healing. Science. 2020;368:186–9.
Tang C, Kamiya T, Liu Y, Kadoki M, Kakuta S, Oshima K, et al. Inhibition of Dectin-1 Signaling Ameliorates Colitis by Inducing Lactobacillus-Mediated Regulatory T-Cell Expansion in the Intestine. Cell Host Microbe. 2015;18:183–97.
Chen L, Wilson JE, Koenigsknecht MJ, Chou WC, Montgomery SA, Truax AD, et al. NLRP12 attenuates colon inflammation by maintaining colonic microbial diversity and promoting protective commensal bacterial growth. Nat Immunol. 2017;18:541–51.
Sun D, Bai R, Zhou W, Yao Z, Liu Y, Tang S, et al. Angiogenin maintains gut microbe homeostasis by balancing alpha-Proteobacteria and Lachnospiraceae. Gut. 2021;70:666–76.
Liu S, Zhang S, Lv X, Lu J, Ren C, Zeng Z, et al. Limonin ameliorates ulcerative colitis by regulating STAT3/miR-214 signaling pathway. Int Immunopharmacol. 2019;75:105768.
Lin K, Zheng W, Guo M, Zhou R, Zhang M, Liu T. The intestinal microbial metabolite acetyl l-carnitine improves gut inflammation and immune homeostasis via CADM2. Biochim Biophys Acta Mol Basis Dis. 2024;1870:167089.
Ranieri R, Ciaglia E, Amodio G, Picardi P, Proto MC, Gazzerro P, et al. N6-isopentenyladenosine dual targeting of AMPK and Rab7 prenylation inhibits melanoma growth through the impairment of autophagic flux. Cell Death Differ. 2018;25:353–67.
Hou Y, Li X, Peng S, Yao J, Bai F, Fang J. Lipoamide Ameliorates Oxidative Stress via Induction of Nrf2/ARE Signaling Pathway in PC12 Cells. J Agric Food Chem. 2019;67:8227–34.
Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–70.
Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–5.
Bao B, et al. Bacterial Sphingolipids Exacerbate Colitis by Inhibiting ILC3-derived IL-22 Production. Cell Mol Gastroenterol Hepatol. 2024;18:101350.
Overacre-Delgoffe AE, Chikina M, Dadey RE, Yano H, Brunazzi EA, Shayan G, et al. Interferon-gamma Drives T(reg) Fragility to Promote Anti-tumor Immunity. Cell. 2017;169:1130–41.e1111.
Battaglia A, Buzzonetti A, Baranello C, Ferrandina G, Martinelli E, Fanfani F, et al. Metastatic tumor cells favor the generation of a tolerogenic milieu in tumor draining lymph node in patients with early cervical cancer. Cancer Immunol Immunother. 2009;58:1363–73.
Wang Y, et al. NRP1 downregulation correlates with enhanced ILC2 responses during IL-33 challenge. Immunology. 2024;172:226-234.
Lennard‒Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2–6.
Zhang W, Lyu M, Bessman NJ, Xie Z, Arifuzzaman M, Yano H, et al. Gut-innervating nociceptors regulate the intestinal microbiota to promote tissue protection. Cell. 2022;185:4170–489.e4120.
Zhou J, Huang S, Wang Z, Huang J, Xu L, Tang X, et al. Targeting EZH2 histone methyltransferase activity alleviates experimental intestinal inflammation. Nat Commun. 2019;10:2427.
Wu X, et al. Group 3 innate lymphoid cells require BATF to regulate gut homeostasis in mice. J Exp Med. 2022;219:e20211861.
Cao Y, Wang X, Yang Q, Deng H, Liu Y, Zhou P, et al. Critical Role of Intestinal Microbiota in ATF3-Mediated Gut Immune Homeostasis. J Immunol. 2020;205:842–52.
Horai R, Zárate-Bladés CR, Dillenburg-Pilla P, Chen J, Kielczewski JL, Silver PB, et al. Microbiota-Dependent Activation of an Autoreactive T-Cell Receptor Provokes Autoimmunity in an Immunologically Privileged Site. Immunity. 2015;43:343–53.
Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:01202.
Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22:598–605.
Seillet C, Luong K, Tellier J, Jacquelot N, Shen RD, Hickey P, et al. The neuropeptide VIP confers anticipatory mucosal immunity by regulating ILC3 activity. Nat Immunol. 2020;21:168–77.
Wen B, Mei Z, Zeng C, Liu S. metaX: a flexible and comprehensive software for processing metabolomics data. BMC Bioinforma. 2017;18:183.
Acknowledgements
This work was supported by the following grants: National Natural Science Foundation of China (No. 81925018, 82130049, 82430055, to J.Z.; 82321001 to Y.Y.; and 82225015 to Q.L.). This work was also supported by the National Key Research and Development Project of China (2021ZD0202400, to Q.L.) and the New Cornerstone Science Foundation through the XPLORER PRIZE (to Q.L.). The authors declare that they have no competing financial interests.
Author information
Authors and Affiliations
Contributions
J.Z. conceived and supervised this study. H.W. cosupervised this study. Y.W. and J.W. performed most of the experiments, analyzed the data, and wrote the manuscript. G.L. participated in most of the experiments. X.Y. performed the bioinformatic analysis. J.W., L. Z. and P. Z. participated in the animal experiments. H.C. collected the samples and clinical information of the IBD patients and paired healthy individuals. Y.F., Y. Y., Q.L., Z.Y. provided suggestions for project design.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests. J.Z. is an editorial board member of Cellular & Molecular Immunology, but she has not been involved in the peer review or the decision-making of the article.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Wang, J., Liu, G. et al. NRP1 instructs IL-17-producing ILC3s to drive colitis progression. Cell Mol Immunol 22, 161–175 (2025). https://doi.org/10.1038/s41423-024-01246-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41423-024-01246-7