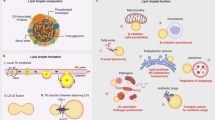
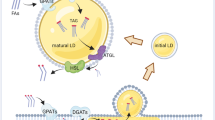
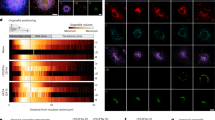
Abstract
Lipid droplets (LDs) are intracellular organelles that can be induced and interact with phagosomes during the process of pathogen phagocytosis in macrophages. However, the function of LDs in phagocytosis remains elusive. Here, we unveil the role of LDs in modulating phagosome formation via a fungal infection model. Specifically, LD accumulation restricted the degree of phagosome formation and protected macrophages from death. Mechanistically, LD formation competitively consumed the intracellular endoplasmic reticulum membrane and altered RAC1 translocation and GTPase activity, which resulted in limited phagosome formation in macrophages during fungal engulfment. Mice with Hilpda-deficient macrophages were more susceptible to the lethal sequelae of systemic infection with C. albicans. Notably, administration of the ATGL inhibitor atglistatin improved host outcomes in disseminated fungal infections. Taken together, our study elucidates the mechanism by which LDs control phagosome formation to prevent immune cell death and offers a potential drug target for the treatment of C. albicans infections.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv13.
Bongomin F, Gago S, Oladele RO, Denning DW. Global and multinational prevalence of fungal diseases-estimate precision. J. Fungi (Basel). 2017;3:57.
Pappas PG, Lionakis MS, Arendrup MC, Ostrosky ZL, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Prim. 2018;4:18026.
Eggimann P, Garbino J, Pittet D. Management of Candida species infections in critically ill patients. Lancet Infect Dis. 2003;3:772–85.
Bustamante CI. Treatment of Candida infection: a view from the trenches! Curr Opin Infect Dis. 2005;18:490–5.
Whaley SG, Berkow EL, Rybak JM, Nishimoto AT, Barker KS, Rogers PD. Azole antifungal resistance in Candida albicans and emerging nonalbicans Candida species. Front Microbiol. 2016;7:2173.
Perlin DS. Mechanisms of echinocandin antifungal drug resistance. Ann NY Acad Sci. 2015;1354:1–11.
Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–91.
van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJ, Cheng SC, Joosten I, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5:329–40.
Lionakis MS, Iliev ID, Hohl TM. Immunity against fungi. JCI Insight. 2017;2:e93156.
Lionakis MS, Drummond RA, Hohl TM. Immune responses to human fungal pathogens and therapeutic prospects. Nat Rev Immunol. 2023;23:433–52.
Gagnon E, Duclos S, Rondeau C, Chevet E, Cameron PH, Steele MO, et al. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell. 2002;110:119–31.
Desjardins M. ER-mediated phagocytosis: a new membrane for new functions. Nat Rev Immunol. 2003;3:280–91.
Niedergang F, Grinstein S. How to build a phagosome: new concepts for an old process. Curr Opin Cell Biol. 2018;50:57–63.
Desjardins M, Huber LA, Parton RG, Griffiths G. Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J Cell Biol. 1994;124:677–88.
Desjardins M, Celis JE, van Meer G, Dieplinger H, Jahraus A, Griffiths G, et al. Molecular characterization of phagosomes. J Biol Chem. 1994;269:32194–200.
Chen T, Feng Y, Sun W, Zhao G, Wu H, Cheng X, et al. The nucleotide receptor STING translocates to the phagosomes to negatively regulate anti-fungal immunity. Immunity. 2023;56:1727–42.e6.
Tucey TM, Verma J, Harrison PF, Snelgrove SL, Lo TL, Scherer AK, et al. Glucose homeostasis is important for immune cell viability during Candida challenge and host survival of systemic fungal infection. Cell Metab. 2018;27:988–1006.e7.
Ding X, Kambara H, Guo R, Kanneganti A, Acosta-Zaldívar M, Li J, et al. Inflammasome-mediated GSDMD activation facilitates escape of Candida albicans from macrophages. Nat Commun 2021;12:6699.
Pol A, Gross SP, Parton RG. Review: biogenesis of the multifunctional lipid droplet: lipids, proteins, and sites. J Cell Biol. 2014;204:635–46.
Pereira-Dutra FS, Bozza PT. Lipid droplets diversity and functions in inflammation and immune response. Expert Rev Proteom. 2021;18:809–25.
Bosch M, Pol A. Eukaryotic lipid droplets: metabolic hubs, and immune first responders. Trends Endocrinol Metab. 2022;33:218–29.
Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol. 2019;20:137–55.
Thiam AR, Beller M. The why, when and how of lipid droplet diversity. J Cell Sci. 2017;130:315–24.
Herker E, Ott M. Emerging role of lipid droplets in host/pathogen interactions. J Biol Chem. 2012;287:2280–7.
Bosch M, Sánchez-Álvarez M, Fajardo A, Kapetanovic R, Steiner B, Dutra F, et al. Mammalian lipid droplets are innate immune hubs integrating cell metabolism and host defense. Science. 2020;370:eaay8085.
Knight M, Braverman J, Asfaha K, Gronert K, Stanley S. Lipid droplet formation in Mycobacterium tuberculosis infected macrophages requires IFN-γ/HIF-1α signaling and supports host defense. PLoS Pathog. 2018;14:e1006874.
van Dierendonck XAMH, de la Rosa Rodriguez MA, Georgiadi A, Mattijssen F, Dijk W, van Weeghel M, et al. HILPDA uncouples lipid droplet accumulation in adipose tissue macrophages from inflammation and metabolic dysregulation. Cell Rep. 2020;30:1811–22.e6.
Maier A, Wu H, Cordasic N, Oefner P, Dietel B, Thiele C, et al. Hypoxia-inducible protein 2 Hig2/Hilpda mediates neutral lipid accumulation in macrophages and contributes to atherosclerosis in apolipoprotein E-deficient mice. FASEB J. 2017;31:4971–84.
Savina A, Vargas P, Guermonprez P, Lennon AM, Amigorena S. Measuring pH, ROS production, maturation, and degradation in dendritic cell phagosomes using cytofluorometry-based assays. Methods Mol Biol. 2010;595:383–402.
Ma X, Tan X, Yu B, Sun W, Wang H, Hu H, et al. DOCK2 regulates antifungal immunity by regulating RAC GTPase activity. Cell Mol Immunol. 2022;19:602–18.
Jia LJ, Rafiq M, Radosa L, Hortschansky P, Cunha C, Cseresnyés Z, et al. Aspergillus fumigatus hijacks human p11 to redirect fungal-containing phagosomes to nondegradative pathway. Cell Host Microbe. 2023;31:373–88.e10.
Millet N, Solis NV, Aguilar D, Lionakis MS, Wheeler RT, Jendzjowsky N, et al. IL-23 signaling prevents ferroptosis-driven renal immunopathology during candidiasis. Nat Commun. 2022;13:5545.
Lionakis MS, Fischer BG, Lim JK, Swamydas M, Wan W, Richard LC, et al. Chemokine receptor Ccr1 drives neutrophil-mediated kidney immunopathology and mortality in invasive candidiasis. PLoS Pathog. 2012;8:e1002865.
MacCallum DM. Massive induction of innate immune response to Candida albicans in the kidney in a murine intravenous challenge model. FEMS Yeast Res. 2009;9:1111–22.
MacCallum DM, Castillo L, Brown Alistair JP, Gow NeilAR, Odds FC. Early-expressed chemokines predict kidney immunopathology in experimental disseminated Candida albicans infections. PLoS One. 2009;4:e6420.
Huo W, Zhang K, Nie Z, Li Q, Jin F. Kidney injury molecule-1 (KIM-1): a novel kidney-specific injury molecule playing potential dual-edged functions in kidney injury. Transpl Rev (Orlando) 2010;24:143–6.
Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072.
Li DD, Jawale CV, Zhou C, Lin L, Trevejo-Nunez GJ, Rahman SA, et al. Fungal sensing enhances neutrophil metabolic fitness by regulating antifungal Glut1 activity. Cell Host Microbe. 2022;30:530–44.e6.
Rossi DC, Figueroa Julio AL, Buesing WR, Candor K, Blancett LT, Evans HM, et al. A metabolic inhibitor arms macrophages to kill intracellular fungal pathogens by manipulating zinc homeostasis. J. Clin. Invest. 2021;131:e147268.
Schrijvers DM, De MG, Herman AG, Martinet W. Phagocytosis in atherosclerosis: molecular mechanisms and implications for plaque progression and stability. Cardiovasc Res 2007;73:470–80.
Chandak PG, Radovic B, Aflaki E, Kolb D, Buchebner M, Fröhlich E, et al. Efficient phagocytosis requires triacylglycerol hydrolysis by adipose triglyceride lipase. J Biol Chem 2010;285:20192–201.
Werb Z, Cohn ZA. Plasma membrane synthesis in the macrophage following phagocytosis of polystyrene latex particles. J Biol Chem 1972;247:2439–46.
Muller WA, Steinman RM, Cohn ZA. The membrane proteins of the vacuolar system. II. bidirectional flow between secondary lysosomes and plasma membrane. J Cell Biol 1980;86:304–14.
Müller-Taubenberger A, Lupas AN, Li H, Ecke M, Simmeth E, Gerisch G. Calreticulin and calnexin in the endoplasmic reticulum are important for phagocytosis. EMBO J. 2001;20:6772–82.
Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med 2001;194:781–95.
van Dierendonck Xanthe AMH, Vrieling F, Smeehuijzen L, Deng L, Boogaard JP, Croes CA, et al. Triglyceride breakdown from lipid droplets regulates the inflammatory response in macrophages. Proc Natl Acad Sci USA. 2022;119:e2114739119.
Zhang F, Zhao M, Braun DR, Ericksen SS, Piotrowski JS, Nelson J, et al. A marine microbiome antifungal targets urgent-threat drug-resistant fungi. Science. 2020;370:974–8.
Zhao X, Guo Y, Jiang C, Chang Q, Zhang S, Luo T, et al. JNK1 negatively controls antifungal innate immunity by suppressing CD23 expression. Nat Med 2017;23:337–46.
Sun W., Ma X., Wang H., Du Y., Chen J., Hu H., et al. MYO1F regulates antifungal immunity by regulating acetylation of microtubules. Proc Natl Acad Sci USA. 2021;118:e2100230118.
Acknowledgements
We thank Dr. Changbin Chen (Institute Pasteur of Shanghai, CAS, Shanghai, China) for kindly providing the C. albicans strain SC5314. This work was supported by grants from the National Natural Science Foundation of China (82321002, 32230033 and 81930039 to CG) and the National Key Research and Development Program of China (2021YFC2300603 and 2023YFC2306102 to CG). This work was also supported by the Future Scholar Program of Shandong University (21510082364125).
Author information
Authors and Affiliations
Contributions
CG and WS conceived and designed the study. WS and HW performed the experiments with assistance from GZ, QS, LZ, XL, and TC; FL, YZ, WZ, XQ, and BL contributed to the discussion and provided reagents. CG and WS analyzed the data and wrote the paper, and TC helped to polish the English writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests. CG is an editorial board member of Cellular & Molecular Immunology, but he has not been involved in the peer review or the decision-making of the article.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, W., Wu, H., Zhao, G. et al. Lipid droplets restrict phagosome formation in antifungal immunity. Cell Mol Immunol 22, 468–484 (2025). https://doi.org/10.1038/s41423-025-01282-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41423-025-01282-x