Abstract
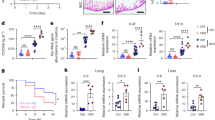
Myeloid-derived suppressor cells (MDSCs) play a protective role against neonatal inflammation during the early postnatal period. However, the mechanisms regulating neonatal MDSC function remain to be fully elucidated. In this study, we report that the bile acid receptor farnesoid X receptor (FXR) acts as a positive regulator of neonatal MDSC function. The FDA-approved FXR agonist obeticholic acid (OCA) protects against neonatal sepsis in an FXR-dependent manner. Genetic deficiency of FXR impairs the immunosuppressive and antibacterial functions of MDSCs, thereby exacerbating the severity of neonatal sepsis. Adoptive transfer of MDSCs alleviates sepsis in both Fxr−/− and Fxrfl/flMrp8-Cre+ pups. Mechanistic studies revealed that Hif1α, a well-established regulator of MDSCs, is a direct transcriptional target of FXR. In patients with neonatal sepsis, downregulation of FXR and HIF-1α in MDSCs was observed, which was inversely correlated with clinical parameters. These observations demonstrate the importance of FXR in neonatal MDSC function and its therapeutic potential in neonatal sepsis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801–10. https://doi.org/10.1001/jama.2016.0287.
Brook B, Harbeson DJ, Shannon CP, Cai B, He D, Ben-Othman R et al. BCG vaccination-induced emergency granulopoiesis provides rapid protection from neonatal sepsis. Sci Transl Med. 2020;12:eaax4517. https://doi.org/10.1126/scitranslmed.aax4517.
Stoll BJ, Puopolo KM, Hansen NI, Sánchez PJ, Bell EF, Carlo WA, et al. Early-Onset Neonatal Sepsis 2015 to 2017, the Rise of Escherichia coli, and the need for novel prevention strategies. JAMA Pediatr. 2020;174:e200593. https://doi.org/10.1001/jamapediatrics.2020.0593.
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–11. https://doi.org/10.1016/S0140-6736(19)32989-7.
Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol. 2021;21:485–98. https://doi.org/10.1038/s41577-020-00490-y.
Yang Z, Huo Y, Zhou S, Guo J, Ma X, Li T, et al. Cancer cell-intrinsic XBP1 drives immunosuppressive reprogramming of intratumoral myeloid cells by promoting cholesterol production. Cell Metab. 2022;34:2018–2035 e2018. https://doi.org/10.1016/j.cmet.2022.10.010.
Cui Z, Xu H, Wu F, Chen J, Zhu L, Shen Z, et al. Maternal circadian rhythm disruption affects neonatal inflammation via metabolic reprograming of myeloid cells. Nat Metab. 2024;6:899–913. https://doi.org/10.1038/s42255-024-01021-y.
Shi M, Chen Z, Chen M, Liu J, Li J, Xing Z, et al. Continuous activation of polymorphonuclear myeloid-derived suppressor cells during pregnancy is critical for fetal development. Cell Mol Immunol. 2021;18:1692–707. https://doi.org/10.1038/s41423-021-00704-w.
He YM, Li X, Perego M, Nefedova Y, Kossenkov AV, Jensen EA, et al. Transitory presence of myeloid-derived suppressor cells in neonates is critical for control of inflammation. Nat Med. 2018;24:224–31. https://doi.org/10.1038/nm.4467.
Derrien M, Mikulic N, Uyoga MA, Chenoll E, Climent E, Howard-Varona A, et al. Gut microbiome function and composition in infants from rural Kenya and association with human milk oligosaccharides. Gut Microbes. 2023;15:2178793. https://doi.org/10.1080/19490976.2023.2178793.
Samara J, Moossavi S, Alshaikh B, Ortega VA, Pettersen VK, Ferdous T, et al. Supplementation with a probiotic mixture accelerates gut microbiome maturation and reduces intestinal inflammation in extremely preterm infants. Cell Host Microbe. 2022;30:696–711.e695. https://doi.org/10.1016/j.chom.2022.04.005.
He Z, Ma Y, Yang S, Zhang S, Liu S, Xiao J, et al. Gut microbiota-derived ursodeoxycholic acid from neonatal dairy calves improves intestinal homeostasis and colitis to attenuate extended-spectrum beta-lactamase-producing enteroaggregative Escherichia coli infection. Microbiome. 2022;10:79. https://doi.org/10.1186/s40168-022-01269-0.
Adorini L, Trauner M. FXR agonists in NASH treatment. J Hepatol. 2023;79:1317–31. https://doi.org/10.1016/j.jhep.2023.07.034.
Clifford BL, Sedgeman LR, Williams KJ, Morand P, Cheng A, Jarrett KE, et al. FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption. Cell Metab. 2021;33:1671–1684.e1674. https://doi.org/10.1016/j.cmet.2021.06.012.
Huang S, Wu Y, Zhao Z, Wu B, Sun K, Wang H, et al. A new mechanism of obeticholic acid on NASH treatment by inhibiting NLRP3 inflammasome activation in macrophage. Metabolism. 2021;120:154797. https://doi.org/10.1016/j.metabol.2021.154797.
Zhang L, Chen J, Yang X, Shen C, Huang J, Zhang D, et al. Hepatic Zbtb18 (Zinc Finger and BTB Domain Containing 18) alleviates hepatic steatohepatitis via FXR (Farnesoid X Receptor). Signal Transduct Target Ther. 2024;9:20. https://doi.org/10.1038/s41392-023-01727-7.
Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen EC, Renooij W, Murzilli S, et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60:463–72. https://doi.org/10.1136/gut.2010.212159.
Li S, Zhuge A, Chen H, Han S, Shen J, Wang K et al. Sedanolide alleviates DSS-induced colitis by modulating the intestinal FXR-SMPD3 pathway in mice. J Adv Res. 2025;69:413–26. https://doi.org/10.1016/j.jare.2024.03.026.
Pi Y, Wu Y, Zhang X, Lu D, Han D, Zhao J, et al. Gut microbiota-derived ursodeoxycholic acid alleviates low birth weight-induced colonic inflammation by enhancing M2 macrophage polarization. Microbiome. 2023;11:19. https://doi.org/10.1186/s40168-022-01458-x.
Panzitt K, Jungwirth E, Krones E, Lee JM, Pollheimer M, Thallinger GG, et al. FXR-dependent Rubicon induction impairs autophagy in models of human cholestasis. J Hepatol. 2020;72:1122–31. https://doi.org/10.1016/j.jhep.2020.01.014.
Hucke S, Herold M, Liebmann M, Freise N, Lindner M, Fleck AK, et al. The farnesoid-X-receptor in myeloid cells controls CNS autoimmunity in an IL-10-dependent fashion. Acta Neuropathol. 2016;132:413–31. https://doi.org/10.1007/s00401-016-1593-6.
Wang XX, Wang D, Luo Y, Myakala K, Dobrinskikh E, Rosenberg AZ, et al. FXR/TGR5 dual agonist prevents progression of nephropathy in diabetes and obesity. J Am Soc Nephrol. 2018;29:118–37. https://doi.org/10.1681/ASN.2017020222.
Kowdley KV, Vuppalanchi R, Levy C, Floreani A, Andreone P, LaRusso NF, et al. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis. J Hepatol. 2020;73:94–101. https://doi.org/10.1016/j.jhep.2020.02.033.
Liu Y, Perego M, Xiao Q, He Y, Fu S, He J, et al. Lactoferrin-induced myeloid-derived suppressor cell therapy attenuates pathologic inflammatory conditions in newborn mice. J Clin Invest. 2019;129:4261–75. https://doi.org/10.1172/JCI128164.
Garcia-Flores V, Romero R, Tarca AL, Peyvandipour A, Xu Y, Galaz J, et al. Deciphering maternal-fetal cross-talk in the human placenta during parturition using single-cell RNA sequencing. Sci Transl Med. 2024;16:eadh8335. https://doi.org/10.1126/scitranslmed.adh8335.
Soisson SM, Parthasarathy G, Adams AD, Sahoo S, Sitlani A, Sparrow C, et al. Identification of a potent synthetic FXR agonist with an unexpected mode of binding and activation. Proc Natl Acad Sci USA. 2008;105:5337–42. https://doi.org/10.1073/pnas.0710981105.
Jiang L, Zhang H, Xiao D, Wei H, Chen Y. Farnesoid X receptor (FXR): Structures and ligands. Comput Struct Biotechnol J. 2021;19:2148–59. https://doi.org/10.1016/j.csbj.2021.04.029.
Weber R, Umansky V. Fighting infant infections with myeloid-derived suppressor cells. J Clin Invest. 2019;129:4080–2. https://doi.org/10.1172/JCI131649.
Shane AL, Sanchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390:1770–80. https://doi.org/10.1016/S0140-6736(17)31002-4.
Strunk T, Molloy EJ, Mishra A, Bhutta ZA. Neonatal bacterial sepsis. Lancet. 2024;404:277–93. https://doi.org/10.1016/S0140-6736(24)00495-1.
Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016;353:i1585. https://doi.org/10.1136/bmj.i1585.
Zampieri FG, Bagshaw SM, Semler MW. Fluid therapy for critically Ill adults with sepsis: a review. JAMA. 2023;329:1967–80. https://doi.org/10.1001/jama.2023.7560.
Abdul-Aziz MH, Hammond NE, Brett SJ, Cotta MO, De Waele JJ, Devaux A, et al. Prolonged vs intermittent infusions of beta-lactam antibiotics in adults with sepsis or septic shock: a systematic review and meta-analysis. JAMA. 2024;332:638–48. https://doi.org/10.1001/jama.2024.9803.
Kumagai M, Kimura A, Takei H, Kurosawa T, Aoki K, Inokuchi T, Matsuishi T. Perinatal bile acid metabolism: bile acid analysis of meconium of preterm and full-term infants. J Gastroenterol. 2007;42:904–10. https://doi.org/10.1007/s00535-007-2108-y.
McNeilly AD, Macfarlane DP, O'Flaherty E, Livingstone DE, Mitić T, McConnell KM, et al. Bile acids modulate glucocorticoid metabolism and the hypothalamic‒pituitary‒adrenal axis in obstructive jaundice. J Hepatol. 2010;52:705–11. https://doi.org/10.1016/j.jhep.2009.10.037.
Cai J, Rimal B, Jiang C, Chiang JYL, Patterson AD. Bile acid metabolism and signaling, the microbiota, and metabolic disease. Pharmacol Ther. 2022;237:108238. https://doi.org/10.1016/j.pharmthera.2022.108238.
Zöhrer E, Resch B, Scharnagl H, Schlagenhauf A, Fauler G, Stojakovic T, et al. Serum bile acids in term and preterm neonates: A case‒control study determining reference values and the influence of early-onset sepsis. Medicine (Baltim). 2016;95:e5219. https://doi.org/10.1097/MD.0000000000005219.
Wang Y, Deng K, Lin P, Huang L, Hu L, Ye J et al. Elevated total bile acid levels as an independent predictor of mortality in pediatric sepsis. Pediatr Res. 2024;1–8. https://doi.org/10.1038/s41390-024-03438-3.
Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–91. https://doi.org/10.1152/physrev.00010.2008.
Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–35. https://doi.org/10.1016/j.cmet.2013.01.003.
Larabi AB, Masson HLP, Baumler AJ. Bile acids as modulators of gut microbiota composition and function. Gut Microbes. 2023;15:2172671. https://doi.org/10.1080/19490976.2023.2172671.
Peiseler M, Schwabe R, Hampe J, Kubes P, Heikenwälder M, Tacke F. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease - novel insights into cellular communication circuits. J Hepatol. 2022;77:1136–60. https://doi.org/10.1016/j.jhep.2022.06.012.
Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J, et al. Bile acid metabolites control T(H)17 and T(reg) cell differentiation. Nature. 2019;576:143–8. https://doi.org/10.1038/s41586-019-1785-z.
You W, Li L, Sun D, Liu X, Xia Z, Xue S, et al. Farnesoid X Receptor Constructs an Immunosuppressive Microenvironment and Sensitizes FXR(high)PD-L1(low) NSCLC to Anti-PD-1 Immunotherapy. Cancer Immunol Res. 2019;7:990–1000. https://doi.org/10.1158/2326-6066.CIR-17-0672.
Glaser F, John C, Engel B, Höh B, Weidemann S, Dieckhoff J, et al. Liver infiltrating T cells regulate bile acid metabolism in experimental cholangitis. J Hepatol. 2019;71:783–92. https://doi.org/10.1016/j.jhep.2019.05.030.
Shi T, Malik A, Yang Vom Hofe A, Matuschek L, Mullen M, Lages CS, et al. Farnesoid X receptor antagonizes macrophage-dependent licensing of effector T lymphocytes and progression of sclerosing cholangitis. Sci Transl Med. 2022;14:eabi4354. https://doi.org/10.1126/scitranslmed.abi4354.
Song X, Sun X, Oh SF, Wu M, Zhang Y, Zheng W, et al. Microbial bile acid metabolites modulate gut RORgamma(+) regulatory T-cell homeostasis. Nature. 2020;577:410–5. https://doi.org/10.1038/s41586-019-1865-0.
Xiahou Z, Wang X, Shen J, Zhu X, Xu F, Hu R, et al. NMI and IFP35 serve as proinflammatory DAMPs during cellular infection and injury. Nat Commun. 2017;8:950. https://doi.org/10.1038/s41467-017-00930-9.
Ulas T, Pirr S, Fehlhaber B, Bickes MS, Loof TG, Vogl T, et al. S100-alarmin-induced innate immune programming protects newborn infants from sepsis. Nat Immunol. 2017;18:622–32. https://doi.org/10.1038/ni.3745.
Liu Y, Binz J, Numerick MJ, Dennis S, Luo G, Desai B, et al. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest. 2003;112:1678–87. https://doi.org/10.1172/JCI18945.
Qin D, Liu S, Lu Y, Yan Y, Zhang J, Cao S, et al. Lgr5 (+) cell fate regulation by coordination of metabolic nuclear receptors during liver repair. Theranostics. 2022;12:6130–42. https://doi.org/10.7150/thno.74194.
Chen L, Jiao T, Liu W, Luo Y, Wang J, Guo X, et al. Hepatic cytochrome P450 8B1 and cholic acid potentiate intestinal epithelial injury in colitis by suppressing intestinal stem cell renewal. Cell Stem Cell. 2022;29:1366–1381.e1369. https://doi.org/10.1016/j.stem.2022.08.008.
Chen J, Wang R, Xiong F, Sun H, Kemper B, Li W et al. Hammerhead-type FXR agonists induce an enhancer RNA Fincor that ameliorates nonalcoholic steatohepatitis in mice. Elife. 2024;13:RP91438. https://doi.org/10.7554/eLife.91438.
Gueders MM, Balbin M, Rocks N, Foidart JM, Gosset P, Louis R, et al. Matrix metalloproteinase-8 deficiency promotes granulocytic allergen-induced airway inflammation. J Immunol. 2005;175:2589–97. https://doi.org/10.4049/jimmunol.175.4.2589.
Chen X, Wu R, Li L, Zeng Y, Chen J, Wei M, et al. Pregnancy-induced changes to the gut microbiota drive macrophage pyroptosis and exacerbate septic inflammation. Immunity. 2023;56:336–352.e339. https://doi.org/10.1016/j.immuni.2023.01.015.
Acknowledgements
This work was supported by the following grants: National Natural Science Foundation of China (No. 82430055, 81925018, and 82130049 to J. Z.; 82001691 to J.H.; and 82488301, 82225015, and 82171284 to Q.L.). This work was also supported by grants to J.H.; the China Postdoctoral Science Foundation (2020M672582); the Science and Technology Planning Project of Guangzhou (No. 2024A03J1237); and the New Cornerstone Science Foundation through the XPLORER PRIZE (to Q.L.).
Author information
Authors and Affiliations
Contributions
J. H., and Y. Z. performed the experiments, analyzed the data, and participated in figure organization and manuscript writing; Y. J., and R. D. participated in most of the experiments. T. L., X. Z. and P. Z. participated in mouse breeding and mouse model construction; K. S. and W. Z. participated in the experiments related to clinical samples; and Q. L. cosupervised this study. J. Z. conceptualized, supervised, and interpreted the experiments and wrote the manuscript with input from all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests. J.Z. is an editorial board member of Cellular & Molecular Immunology, but she has not been involved in the peer review or the decision-making of the article.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, J., Zhang, Y., Jing, Y. et al. FXR protects against neonatal sepsis by enhancing the immunosuppressive function of MDSCs. Cell Mol Immunol 22, 661–673 (2025). https://doi.org/10.1038/s41423-025-01289-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41423-025-01289-4
Keywords
This article is cited by
-
Functional importance of bile acid-FXR signaling in neonatal immunity and disease
Cellular & Molecular Immunology (2025)