Abstract
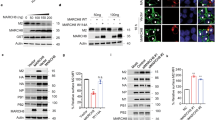
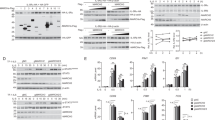
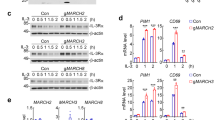
T-cell immunoglobulin mucin family member-1 (TIM-1, also known as HAVCR1/KIM-1) is a transmembrane glycoprotein that has been reported to act as an entry receptor for multiple flaviviruses including Zika virus (ZIKV). The post-translational regulation of TIM-1 and its effects on ZIKV infection are unclear. In this study, we identified the membrane-associated RING-CH-type finger (MARCH) E3 ubiquitin ligase family members MARCH2 and MARCH3 as critical negative regulators of TIM-1 under physiological conditions. MARCH2 and MARCH3 associate with TIM-1 and mediate its K48-linked polyubiquitination at K338 and K346 respectively, leading to subsequent proteasomal degradation. While deficiency of either MARCH2 or MARCH3 modestly increases TIM-1 levels and enhances ZIKV infectivity, double knockout of MARCH2/3 has a more dramatic effect. Double knockout of MARCH2/3 increased ZIKV infectivity in wild-type but not TIM-1 knockout cells, and reconstitution of TIM-1K338R/K346R into TIM-1-deficient cells increases ZIKV infectivity to a higher degree than reconstitution with wild-type TIM-1. Knockout of either MARCH2 or MARCH3 increased ZIKV infectivity and pathogenesis in mice, whereas double knockout of MARCH2/3 has a more dramatic effect. These findings suggest that MARCH2 and MARCH3 target TIM-1 for K48-linked polyubiquitination and proteasomal degradation, thereby acting as redundant host restriction factors to limit ZIKV infection and pathogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All study data are included in the article and/or supplementary materials.
References
Liu Z-Y, Shi W-F, Qin C-F. The evolution of Zika virus from Asia to the Americas. Nat Rev Microbiol. 2019;17:131–9.
Pierson TC, Diamond MS. The emergence of Zika virus and its new clinical syndromes. Nature. 2018;560:573–81.
Lessler J, Chaisson LH, Kucirka LM, Bi Q, Grantz K, Salje H, et al. Assessing the global threat from Zika virus. Science. 2016;353:aaf8160.
Mehand MS, Al-Shorbaji F, Millett P, Murgue B. The WHO R&D Blueprint: 2018 review of emerging infectious diseases requiring urgent research and development efforts. Antiviral Res. 2018;159:63–67.
Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–44.
Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–42.
Li M, Ablan SD, Miao C, Zheng YM, Fuller MS, Rennert PD, et al. TIM-family proteins inhibit HIV-1 release. Proc Natl Acad Sci USA. 2014;111:E3699–3707.
Bailly V, Zhang Z, Meier W, Cate R, Sanicola M, Bonventre JV, et al. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem. 2002;277:39739–48.
Bod L, Kye YC, Shi J, Torlai Triglia E, Schnell A, Fessler J, et al. B-cell-specific checkpoint molecules that regulate anti-tumour immunity. Nature. 2023;619:348–56.
Giraldo MI, Xia H, Aguilera-Aguirre L, Hage A, van Tol S, Shan C, et al. Envelope protein ubiquitination drives entry and pathogenesis of Zika virus. Nature. 2020;585:414–9.
Liu J, Quan Y, Tong H, Zhu Y, Shi X, Liu Y, et al. Insights into mosquito-borne arbovirus receptors. Cell Insight. 2024;3:100196.
Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, et al. Biology of Zika Virus Infection in Human Skin Cells. J Virol. 2015;89:8880–96.
Yu W, Tao J, Cao H, Zheng W, Zhang B, Zhang Y, et al. The HAVCR1-centric host factor network drives Zika virus vertical transmission. Cell Rep. 2025;44:115464.
Kondratowicz AS, Lennemann NJ, Sinn PL, Davey RA, Hunt CL, Moller-Tank S, et al. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburgvirus. Proc Natl Acad Sci USA. 2011;108:8426–31.
Jemielity S, Wang JJ, Chan YK, Ahmed AA, Li W, Monahan S, et al. TIM-family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine. PLoS Pathog. 2013;9:e1003232.
Lin H, Li S, Shu HB. The membrane-associated MARCH E3 ligase family: emerging roles in immune regulation. Front Immunol. 2019;10:1751.
Zheng C. The emerging roles of the MARCH ligases in antiviral innate immunity. Int J Biol Macromol. 2021;171:423–7.
Chen R, Li M, Zhang Y, Zhou Q, Shu HB. The E3 ubiquitin ligase MARCH8 negatively regulates IL-1beta-induced NF-kappaB activation by targeting the IL1RAP coreceptor for ubiquitination and degradation. Proc Natl Acad Sci USA. 2012;109:14128–33.
Lin H, Gao D, Hu MM, Zhang M, Wu XX, Feng L, et al. MARCH3 attenuates IL-1beta-triggered inflammation by mediating K48-linked polyubiquitination and degradation of IL-1RI. Proc Natl Acad Sci USA. 2018;115:12483–8.
Lin H, Feng L, Cui KS, Zeng LW, Gao D, Zhang LX, et al. The membrane-associated E3 ubiquitin ligase MARCH3 downregulates the IL-6 receptor and suppresses colitis-associated carcinogenesis. Cell Mol Immunol. 2021;18:2648–59.
Zeng LW, Feng L, Liu R, Lin H, Shu HB, Li S. The membrane-associated ubiquitin ligases MARCH2 and MARCH3 target IL-5 receptor alpha to negatively regulate eosinophilic airway inflammation. Cell Mol Immunol. 2022;19:1117–29.
Liu R, Zeng LW, Li HF, Shi JG, Zhong B, Shu HB, et al. PD-1 signaling negatively regulates the common cytokine receptor gamma chain via MARCH5-mediated ubiquitination and degradation to suppress anti-tumor immunity. Cell Res. 2023;33:923–39.
Umthong S, Timilsina U, D’Angelo MR, Salka K, Stavrou S. MARCH2, a T cell specific factor that restricts HIV-1 infection. PLoS Pathog. 2024;20:e1012330.
Feng L, Li C, Zeng LW, Gao D, Sun YH, Zhong L, et al. MARCH3 negatively regulates IL-3-triggered inflammatory response by mediating K48-linked polyubiquitination and degradation of IL-3Ralpha. Sig Transduct Target Ther. 2022;7:21.
Gao D, Yi XM, Feng L, Li S, Shu HB. MARCH8 mediates K27-linked polyubiquitination of IL-7 receptor alpha to negatively regulate IL-7-triggered T cell homeostasis. J Immunol. 2024;213:1467–78.
Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11:783–4.
Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–87.
Chathuranga K, Kim TH, Lee H, Park JS, Kim JH, Chathuranga WAG, et al. Negative regulation of NEMO signaling by the ubiquitin E3 ligase MARCH2. EMBO J. 2020;39:e105139.
Nakamura N. The role of the transmembrane RING finger proteins in cellular and organelle function. Membranes. 2011;1:354–93.
Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, et al. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe. 2016;20:155–66.
Dejarnac O, Hafirassou ML, Chazal M, Versapuech M, Gaillard J, Perera-Lecoin M, et al. TIM-1 ubiquitination mediates dengue virus entry. Cell Rep. 2018;23:1779–93.
Wang J, Jiang B, Wang K, Dai J, Dong C, Wang Y, et al. A cathelicidin antimicrobial peptide from Hydrophis cyanocinctus inhibits Zika virus infection by downregulating expression of a viral entry factor. J Biol Chem. 2022;298:102471.
Fukuda H, Nakamura N, Hirose S. MARCH-III is a novel component of endosomes with properties similar to those of MARCH-II. Journal Biochem. 2006;139:137–45.
Dukhovny A, Lamkiewicz K, Chen Q, Fricke M, Jabrane-Ferrat N, Marz M, et al. A CRISPR activation screen identifies genes that protect against Zika virus infection. Journal Virol. 2019;93:e00211–19.
Yang C, Xu H, Yang D, Xie Y, Xiong M, Fan Y, et al. A renal YY1-KIM1-DR5 axis regulates the progression of acute kidney injury. Nat Commun. 2023;14:4261.
Umetsu SE, Lee WL, McIntire JJ, Downey L, Sanjanwala B, Akbari O, et al. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat Immunol. 2005;6:447–54.
de Souza AJ, Oriss TB, O’Malley KJ, Ray A, Kane LP. T cell Ig and mucin 1 (TIM-1) is expressed on in vivo-activated T cells and provides a costimulatory signal for T cell activation. Proc Natl Acad Sci USA. 2005;102:17113–8.
Wang J, Qiao L, Hou Z, Luo G. TIM-1 promotes Hepatitis C virus cell attachment and infection. J Virol. 2017;91:e01583–16.
Zhang X, Liang C, Wang H, Guo Z, Rong H, Pan J, et al. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a functional entry factor for tick-borne encephalitis virus. Mbio. 2022;13:e0286021.
Yi X-M, Lei Y-L, Li M, Zhong L, Li S. The monkeypox virus-host interplays. Cell Insight. 2024;3:100185.
Zhao C, Chen J, Liu Z, Liang H, Chen X, Cheng L, et al. Activation of nicotinic acetylcholine receptor alpha7 subunit limits Zika viral infection via promoting autophagy and ferroptosis. Mol Ther. 2024;32:2641–61.
Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints12. Am J Epidemiol. 1938;27:493–7.
Acknowledgements
We thank Wen-Hua Xu, Xu Chen, and other members of our laboratory for technical help and discussions. This work was supported by grants from the State Key R&D Program of China (2024YFA1306500, 2022YFA1304900), the National Natural Science Foundation of China (32188101), the Major Project of Guangzhou National Laboratory (GZNL2024A01014, GZNL2024A01016), the Fundamental Research Funds for the Central Universities (2042022dx0003), and Natural Science Foundation of Wuhan (2024040701010031).
Author information
Authors and Affiliations
Contributions
QZ, SL, and H-BS designed research; QZ, Z-WM, H-FL, and J-QZ performed research; QZ, SL, and H-BS analyzed data; and QZ, SL, and H-BS wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests. Dr. H.-B.S. is editorial board member of Cellular & Molecular Immunology, but he has not been involved in the peer review or the decision-making of the article.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Q., Ma, ZW., Li, HF. et al. The membrane-associated ubiquitin ligases MARCH2 and MARCH3 target TIM-1 to limit Zika virus infection. Cell Mol Immunol 22, 1032–1044 (2025). https://doi.org/10.1038/s41423-025-01334-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41423-025-01334-2
Key Words
This article is cited by
-
MARCH to combat Zika virus infection
Cellular & Molecular Immunology (2025)