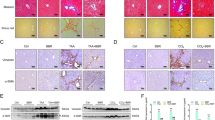
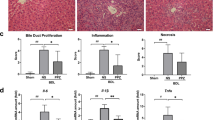
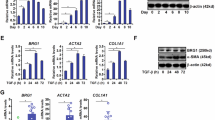
Abstract
Gut-derived metabolites are essential for liver fibrogenesis. The aim of this study was to determine the alteration of indole-3-propionic acid (IPA), a crucial tryptophan metabolite, in liver fibrosis and delineate the roles of enterogenic IPA in fibrogenesis. In the present study, metabolomics assays focused on tryptophan metabolism were applied to explore the decreased levels of IPA in the feces and serum of cirrhotic patients, as well as in the feces and portal vein serum of fibrotic mice. Oral IPA administration exerted strong antifibrotic effects with favorable biosafety in three fibrotic models via multicellular modulation. Multiplex immunohistochemical staining and DAOSLIMIT imaging demonstrated that gut-derived IPA was directly captured by hepatic macrophages. Macrophage-specific AhR knockout blocked the antifibrotic effect of IPA, while the therapeutic efficacy was maintained in mice with HSC- or hepatocyte-specific AhR depletion. Furthermore, IPA governed macrophage recruitment, S100A8/A9+ phenotype transformation and profibrotic and proinflammatory functions, resulting in amelioration of hepatic fibrogenesis. Mechanistically, IPA targeted the AhR/NF-κB/S100A8/A9 axis and AhR/SPHK2/S1P signaling to inhibit the profibrotic biological characteristics of macrophages and subsequently interrupted the profibrogenic crosstalk between macrophages and hepatic stellate cells (HSCs) in coculture systems and 3D liver spheroid models. These findings increase the understanding of the effects of enterogenic tryptophan metabolites on liver fibrogenesis via the gut‒liver axis and support the translational potential of IPA. By targeting profibrogenic macrophages, IPA could serve as a promising candidate for the clinical management of liver fibrosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All sequencing data generated during this study have been deposited in the Gene Expression Omnibus (GEO) database (GSE277148). The previously published human scRNA-seq datasets used in this study are available in GSE168933. The metagenomic and metabolomic data, along with the research materials referenced in this article, are available from the corresponding author upon reasonable request.
Code availability
All sequencing data generated during this study have been deposited in the Gene Expression Omnibus (GEO) database (GSE277148). The previously published human scRNA-seq datasets used in this study are available in GSE168933. The metagenomic and metabolomic data, along with the research materials referenced in this article, are available from the corresponding author upon reasonable request.
References
Huang DQ, Terrault NA, Tacke F, Gluud LL, Arrese M, Bugianesi E, et al. Global epidemiology of cirrhosis - etiology, trends and predictions. Nat Rev Gastroenterol Hepatol. 2023;20:388–98.
Trivedi P, Wang S, Friedman SL. The power of plasticity-metabolic regulation of hepatic stellate cells. Cell Metab. 2021;33:242–57.
Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151–66.
Ma PF, Gao CC, Yi J, Zhao JL, Liang SQ, Zhao Y, et al. Cytotherapy with M1-polarized macrophages ameliorates liver fibrosis by modulating immune microenvironment in mice. J Hepatol. 2017;67:770–9.
Hsu CL, Schnabl B. The gut-liver axis and gut microbiota in health and liver disease. Nat Rev Microbiol. 2023;21:719–33.
Guan H, Zhang X, Kuang M, Yu J. The gut-liver axis in immune remodeling of hepatic cirrhosis. Front Immunol. 2022;13:946628.
Agus A, Planchais J, Sokol H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe. 2018;23:716–24.
Wang G, Fan Y, Zhang G, Cai S, Ma Y, Yang L, et al. Microbiota-derived indoles alleviate intestinal inflammation and modulate microbiome by microbial cross-feeding. Microbiome. 2024;12:59.
Jia D, Wang Q, Qi Y, Jiang Y, He J, Lin Y, et al. Microbial metabolite enhances immunotherapy efficacy by modulating T cell stemness in pancancer. Cell. 2024;187:1651–65.e21.
Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551:648–52.
Flannigan KL, Nieves KM, Szczepanski HE, Serra A, Lee JW, Alston LA, et al. The Pregnane X Receptor and Indole-3-Propionic Acid Shape the Intestinal Mesenchyme to Restrain Inflammation and Fibrosis. Cell Mol Gastroenterol Hepatol. 2023;15:765–95.
Sehgal R, Ilha M, Vaittinen M, Kaminska D, Männistö V, Kärjä V, et al. Indole-3-Propionic acid, a gut-derived tryptophan metabolite, associates with hepatic fibrosis. Nutrients. 2021;13:3509.
Wang S, Xiong L, Ruan Z, Gong X, Luo Y, Wu C, et al. Indole-3-propionic acid alleviates sepsis-associated acute liver injury by activating pregnane X receptor. Mol Med. 2023;29:65.
Min BH, Devi S, Kwon GH, Gupta H, Jeong JJ, Sharma SP, et al. Gut microbiota-derived indole compounds attenuate metabolic dysfunction-associated steatotic liver disease by improving fat metabolism and inflammation. Gut Microbes. 2024;16:2307568.
Ganesan R, Gupta H, Jeong JJ, Sharma SP, Won SM, Oh KK, et al. Characteristics of microbiome-derived metabolomics according to the progression of alcoholic liver disease. Hepatol Int. 2024;18:486–99.
Zhao ZH, Xin FZ, Xue Y, Hu Z, Han Y, Ma F, et al. Indole-3-propionic acid inhibits gut dysbiosis and endotoxin leakage to attenuate steatohepatitis in rats. Exp Mol Med. 2019;51:1–14.
Ilha M, Sehgal R, Matilainen J, Rilla K, Kaminska D, Gandhi S, et al. Indole-3-propionic acid promotes hepatic stellate cells inactivation. J Transl Med. 2025;23:253.
Yuan X, Yang J, Huang Y, Li J, Li Y. Gut Microbiota metabolite 3-indolepropionic acid directly activates hepatic stellate cells by ROS/JNK/p38 signaling pathways. Biomolecules. 2023;13:1464.
Liu F, Sun C, Chen Y, Du F, Yang Y, Wu G. Indole-3-propionic Acid-aggravated CCl(4)-induced Liver Fibrosis via the TGF-β1/Smads signaling pathway. J Clin Transl Hepatol. 2021;9:917–30.
Wei Y, Peng N, Deng C, Zhao F, Tian J, Tang Y, et al. Aryl hydrocarbon receptor activation drives polymorphonuclear myeloid-derived suppressor cell response and efficiently attenuates experimental Sjögren’s syndrome. Cell Mol Immunol. 2022;19:1361–72.
Dutta M, Lim JJ, Cui JY. Pregnane X receptor and the gut-liver axis: a recent update. Drug Metab Dispos. 2022;50:478–91.
Unamuno X, Gómez-Ambrosi J, Ramírez B, Rodríguez A, Becerril S, Valentí V, et al. NLRP3 inflammasome blockade reduces adipose tissue inflammation and extracellular matrix remodeling. Cell Mol Immunol. 2021;18:1045–57.
Németh J, Stein I, Haag D, Riehl A, Longerich T, Horwitz E, et al. S100A8 and S100A9 are novel nuclear factor kappa B target genes during malignant progression of murine and human liver carcinogenesis. Hepatology. 2009;50:1251–62.
Kuo A, Hla T. Regulation of cellular and systemic sphingolipid homeostasis. Nat Rev Mol Cell Biol. 2024;25:802–21.
Yang L, Yue S, Yang L, Liu X, Han Z, Zhang Y, et al. Sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis is involved in liver fibrosis-associated angiogenesis. J Hepatol. 2013;59:114–23.
Buonomo EL, Mei S, Guinn SR, Leo IR, Peluso MJ, Nolan MA, et al. Liver stromal cells restrict macrophage maturation and stromal IL-6 limits the differentiation of cirrhosis-linked macrophages. J Hepatol. 2022;76:1127–37.
Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66:1300–12.
Masoodi M, Gastaldelli A, Hyötyläinen T, Arretxe E, Alonso C, Gaggini M, et al. Metabolomics and lipidomics in NAFLD: biomarkers and noninvasive diagnostic tests. Nat Rev Gastroenterol Hepatol. 2021;18:835–56.
Wrzosek L, Ciocan D, Hugot C, Spatz M, Dupeux M, Houron C, et al. Microbiota tryptophan metabolism induces aryl hydrocarbon receptor activation and improves alcohol-induced liver injury. Gut. 2021;70:1299–308.
Sehgal R, de Mello VD, Männistö V, Lindström J, Tuomilehto J, Pihlajamäki J, et al. Indolepropionic Acid, a Gut Bacteria-produced tryptophan metabolite and the risk of type 2 diabetes and non-alcoholic fatty liver disease. Nutrients. 2022;14:4695.
Sharma SP, Gupta H, Kwon GH, Lee SY, Song SH, Kim JS, et al. Gut microbiome and metabolome signatures in liver cirrhosis-related complications. Clin Mol Hepatol. 2024;30:845–62.
Yisireyili M, Takeshita K, Saito S, Murohara T, Niwa T. Indole-3-propionic acid suppresses indoxyl sulfate-induced expression of fibrotic and inflammatory genes in proximal tubular cells. Nagoya J Med Sci. 2017;79:477–86.
Zhao Z, Jiang S, Fan Q, Xu K, Xu Y, Wu F, et al. Apocynum venetum leaf extract alleviated doxorubicin-induced cardiotoxicity by regulating organic acid metabolism in gut microbiota. Front Pharm. 2023;14:1286210.
Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411.
Hammerich L, Tacke F. Hepatic inflammatory responses in liver fibrosis. Nat Rev Gastroenterol Hepatol. 2023;20:633–46.
Hou C, Wang D, Zhao M, Ballar P, Zhang X, Mei Q, et al. MANF brakes TLR4 signaling by competitively binding S100A8 with S100A9 to regulate macrophage phenotypes in hepatic fibrosis. Acta Pharm Sin B. 2023;13:4234–52.
Liu Y, Kong X, You Y, Xiang L, Zhang Y, Wu R, et al. S100A8-Mediated NLRP3 Inflammasome-dependent pyroptosis in macrophages facilitates liver fibrosis progression. Cells. 2022;11:3579.
Tao L, Zhang Q, Liu L, Wang K, Wang J, Liu X, et al. Inhibition of AhR disrupts intestinal epithelial barrier and induces intestinal injury by activating NF-κB in COPD. Faseb J. 2024;38:e70256.
Kimura A, Naka T, Nakahama T, Chinen I, Masuda K, Nohara K, et al. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J Exp Med. 2009;206:2027–35.
Ishay Y, Nachman D, Khoury T, Ilan Y. The role of the sphingolipid pathway in liver fibrosis: an emerging new potential target for novel therapies. Am J Physiol Cell Physiol. 2020;318:C1055–c64.
González-Fernández B, Sánchez DI, González-Gallego J, Tuñón MJ. Sphingosine 1-phosphate signaling as a target in hepatic fibrosis therapy. Front Pharm. 2017;8:579.
Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology. 2017;66:1727–38.
Mohamad Nor MH, Ayob N, Mokhtar NM, Raja Ali RA, Tan GC, Wong Z, et al. The Effect of Probiotics (MCP(®) BCMC(®) Strains) on Hepatic Steatosis, small intestinal mucosal immune function, and intestinal barrier in patients with non-alcoholic fatty liver disease. Nutrients. 2021;13:3192.
Sayegh M, Ni QQ, Ranawana V, Raikos V, Hayward NJ, Hayes HE, et al. Habitual consumption of high-fiber bread fortified with bean hulls increased plasma indole-3-propionic concentration and decreased putrescine and deoxycholic acid fecal concentrations in healthy volunteers. Br J Nutr. 2023;130:1521–36.
Mercenier A, Vu LD, Poppe J, Albers R, McKay S, Van den Abbeele P. Carrot-Derived Rhamnogalacturonan-I Consistently Increases the Microbial Production of Health-Promoting Indole-3-Propionic Acid Ex Vivo. Metabolites. 2024;14:722.
Kim CS, Jung S, Hwang GS, Shin DM. Gut microbiota indole-3-propionic acid mediates neuroprotective effect of probiotic consumption in healthy elderly: A randomized, double-blind, placebo-controlled, multicenter trial and in vitro study. Clin Nutr. 2023;42:1025–33.
Wang Y, Zhou J, Ye J, Sun Z, He Y, Zhao Y, et al. Multiomics reveal microbial determinants impacting the treatment outcome of antidepressants in major depressive disorder. Microbiome. 2023;11:195.
Wei J, Sun Y, Wang H, Zhu T, Li L, Zhou Y, et al. Designer cellular spheroids with DNA origami for drug screening. Sci Adv. 2024;10:eado9880.
Wu J, Lu Z, Jiang D, Guo Y, Qiao H, Zhang Y, et al. Iterative tomography with digital adaptive optics permits hour-long intravital observation of 3D subcellular dynamics at millisecond scale. Cell. 2021;184:3318–32.e17.
Lu Z, Cai Y, Nie Y, Yang Y, Wu J, Dai Q. A practical guide to scanning light-field microscopy with digital adaptive optics. Nat Protoc. 2022;17:1953–79.
Lu Z, Wu J, Qiao H, Zhou Y, Yan T, Zhou Z, et al. Phase-space deconvolution for light field microscopy. Opt Express. 2019;27:18131–45.
Lu Z, Zuo S, Shi M, Fan J, Xie J, Xiao G, et al. Long-term intravital subcellular imaging with confocal scanning light-field microscopy. Nat Biotechnol. 2025;43:569–80.
Lu Z, Liu Y, Jin M, Luo X, Yue H, Wang Z, et al. Virtual-scanning light-field microscopy for robust snapshot high-resolution volumetric imaging. Nat Methods. 2023;20:735–46.
Acknowledgements
This work was supported by the National Key R&D Program of China (2023YFC2507500), the National Natural Science Foundation of China (82470661, 82070616, 82270636, 82100608, 82300607), the New Quality Clinical Specialties of High-end Medical Disciplinary Construction in Pudong New Area (2025-PWXZ-04) and the Talent Plan of the Shanghai Municipal Health Commission for Academic Leaders (2022XD028). We gratefully acknowledge Eastern Hepatobiliary Surgery Hospital (Navy Medical University, Shanghai, China) for supporting tissue sample determination. Figure support was provided by Figdraw.
Author information
Authors and Affiliations
Contributions
Xin Zeng and Yong Lin: designed, coordinated and supervised the study, reviewed, and edited the manuscript; Yuanyuan Luo, Yarong Hao, Chunyan Sun, Zhi Lu, and Hao Wang: original draft preparation, established the animal models, and drafted the manuscript; Yuhan Lin, Lingyan Cai, Chenhong Ding, Binbin Li, and Fei Chen, and Yiting Lu: performed the data analysis and interpretation of the data. All the authors critically revised the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study protocol was conducted in accordance with the Declaration of Helsinki and approved by the Shanghai Changzheng Hospital Ethics Committee (no. 2018SL004). Written informed consent was obtained from all participants before recruitment. All the animal studies were approved by the Shanghai East Hospital Ethics Committee (no. 2023083) and conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC), ensuring the welfare and minimal suffering of the animals involved.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, Y., Hao, Y., Sun, C. et al. Gut-derived indole propionic acid alleviates liver fibrosis by targeting profibrogenic macrophages via the gut‒liver axis. Cell Mol Immunol 22, 1414–1426 (2025). https://doi.org/10.1038/s41423-025-01339-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41423-025-01339-x