Abstract
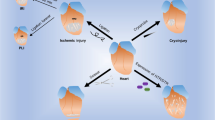
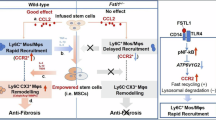
The regenerative capacities of organs in adult mammals vary significantly. Unlike the liver, which possesses remarkable regenerative potential, the repair of cardiac injuries has long posed a critical medical challenge. Recent studies have highlighted the pivotal role of the immune microenvironment in repairing damage in these tissues, but the key cell types and their mechanisms of action remain incompletely understood. In this study, we established a model of concurrent physical trauma to the hearts and livers of adult mice, revealing that these two injured tissues drive distinct immune microenvironments. The liver primarily accumulates lymphocytes, whereas the heart recruits macrophages and neutrophils. Notably, CD160+CD8+ intraepithelial lymphocytes in the liver were found to suppress fibrosis postliver injury and mitigate cardiac fibrosis when delivered via hydrogel patches. Conversely, in response to heart trauma, recruited inflammatory macrophages not only express proinflammatory cytokines but also coexpress CCRL2. While CCRL2 did not directly alter the intensity of the inflammatory response, it facilitated fibroblast proliferation and migration through its interaction with Na+/K+-ATPase on fibroblasts. These findings elucidated the contrasting immune microenvironments between the heart and liver following injury and provided novel insights and strategies for diagnosing and treating cardiac diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017;356:1026–30.
Baddour JA, Sousounis K, Tsonis PA. Organ repair and regeneration: an overview. Birth Defects Res Part C Embryo Today Rev. 2012;96:1–29.
Campana L, Esser H, Huch M, Forbes S. Liver regeneration and inflammation: from fundamental science to clinical applications. Nat Rev Mol Cell Biol. 2021;22:608–24.
Michalopoulos GK, Bhushan B. Liver regeneration: biological and pathological mechanisms and implications. Nat Rev Gastroenterol Hepatol. 2021;18:40–55.
Kim W, et al. Hippo signaling interactions with Wnt/β-catenin and Notch signaling repress liver tumorigenesis. J Clin Investig. 2017;127:137–52.
Huang R, Zhang X, Gracia-Sancho J, Xie W-F. Liver regeneration: cellular origin and molecular mechanisms. Liver Int. 2022;42:1486–95.
Hoffmann K, et al. Markers of liver regeneration-the role of growth factors and cytokines: a systematic review. BMC Surg. 2020;20:31.
Zhang C, Sun C, Zhao Y, Ye B, Yu G. Signaling pathways of liver regeneration: biological mechanisms and implications. iScience. 2024;27:108683.
Hora S, Wuestefeld T. Liver injury and regeneration: current understanding, new approaches, and future perspectives. Cells 2023;12:2129.
Nguyen-Lefebvre AT, et al. Modeling the liver’s regenerative capacity across different clinical conditions. JHEP Rep Innov Hepatol. 2025;7:101465.
Cahill TJ, Choudhury RP, Riley PR. Heart regeneration and repair after myocardial infarction: translational opportunities for novel therapeutics. Nat Rev Drug Discov. 2017;16:699–717.
Lerman DA, Alotti N, Ume KL, Péault B. Cardiac repair and regeneration: the value of cell therapies. Eur Cardiol. 2016;11:43–48.
Frangogiannis NG. The extracellular matrix in myocardial injury, repair, and remodeling. J Clin Investig. 2017;127:1600–12.
Xue M, Jackson CJ. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care. 2015;4:119–36.
Schütte JP, et al. Cell type–specific secretome analysis reveals liver-heart crosstalk in HFpEF. Circ Res. 2025;136:1516–8.
Sun H, et al. Cirrhosis promotes cardiac fibrosis development by inhibiting Notch1 in cardiac fibroblasts. JACC Basic Transl Sci. 2025;10:612–31.
Gieseck RL, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18:62–76.
Julier Z, Park AJ, Briquez PS, Martino MM. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017;53:13–28.
Rurik JG, Aghajanian H, Epstein JA. Immune cells and immunotherapy for cardiac injury and repair. Circ Res. 2021;128:1766–79.
Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol. 2015;15:117–29.
Yap J, et al. Macrophages in cardiac remodeling after myocardial infarction. Nat Rev Cardiol. 2023;20:373–85.
de Couto G. Macrophages in cardiac repair: environmental cues and therapeutic strategies. Exp Mol Med. 2019;51:1–10.
Yu Y, et al. Macrophages play a key role in tissue repair and regeneration. PeerJ. 2022;10:e14053.
Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181–94.
Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17:306–21.
Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62.
Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86:513–28.
Gomes RN, Manuel F, Nascimento DS. The bright side of fibroblasts: molecular signature and regenerative cues in major organs. npj Regen Med. 2021;6:43.
Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151–66.
Ivey MJ, Tallquist MD. Defining the cardiac fibroblast. Circ J. 2016;80:2269–76.
Van Linthout S, Miteva K, Tschöpe C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res. 2014;102:258–69.
Huang E, et al. The roles of immune cells in the pathogenesis of fibrosis. Int J Mol Sci. 2020;21:5203.
Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–57.
Budi EH, Schaub JR, Decaris M, Turner S, Derynck R. TGF-β as a driver of fibrosis: physiological roles and therapeutic opportunities. J Pathol. 2021;254:358–73.
Waterhouse NJ, et al. A central role for bid in granzyme B-induced apoptosis *. J Biol Chem. 2005;280:4476–82.
Velotti F, Barchetta I, Cimini FA, Cavallo MG. Granzyme B in inflammatory diseases: apoptosis, inflammation, extracellular matrix remodeling, epithelial-to-mesenchymal transition and fibrosis. Front Immunol. 2020;11:587581.
Epelman S, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104.
Li G, et al. Optogenetic vagal nerve stimulation attenuates heart failure by limiting the generation of monocyte-derived inflammatory CCRL2+ macrophages. Immunity. 2025;58:1847–1861.e1849.
McLellan MA, et al. High-resolution transcriptomic profiling of the heart during chronic stress reveals cellular drivers of cardiac fibrosis and hypertrophy. Circulation. 2020;142:1448–63.
Pierre SV, Xie Z. The Na,K-ATPase receptor complex. Cell Biochem Biophys. 2006;46:303–15.
Shoshani L, et al. The polarized expression of Na+,K+-ATPase in epithelia depends on the association between β-subunits located in neighboring cells. Mol Biol Cell. 2005;16:1071–81.
Tokhtaeva E, et al. Epithelial junctions depend on intercellular trans-interactions between the Na,K-ATPase β₁ subunits. J Biol Chem. 2011;286:25801–12.
Rajasekaran SA, et al. Na-K-ATPase regulates tight junction permeability through occludin phosphorylation in pancreatic epithelial cells. Am J Physiol-Gastrointest Liver Physiol. 2007;292:G124–G133.
Rajasekaran SA, et al. Na,K-ATPase activity is required for formation of tight junctions, desmosomes, and induction of polarity in epithelial cells. Mol Biol Cell. 2001;12:3717–32.
Rajasekaran SA, Rajasekaran AK. Na,K-ATPase and epithelial tight junctions. FBL. 2009;14:2130–48.
Kikuchi K, Poss KD. Cardiac regenerative capacity and mechanisms. Annu Rev Cell Dev Biol. 2012;28:719–41.
Garbern JC, Lee RT. Heart regeneration: 20 years of progress and renewed optimism. Dev Cell. 2022;57:424–39.
Gong R, Jiang Z, Zagidullin N, Liu T, Cai B. Regulation of cardiomyocyte fate plasticity: a key strategy for cardiac regeneration. Signal Transduct Target Ther. 2021;6:31.
Abu Rmilah A, et al. Understanding the marvels behind liver regeneration. Wiley Interdiscip Rev Dev Biol. 2019;8:e340.
Crowl JT, et al. Tissue-resident memory CD8+ T cells possess unique transcriptional, epigenetic and functional adaptations to different tissue environments. Nat Immunol. 2022;23:1121–31.
Masopust D, Soerens AG. Tissue-resident T cells and other resident leukocytes. Ann Rev Immunol. 2019;37:521–46.
Hiebert PR, Wu D, Granville DJ. Granzyme B degrades extracellular matrix and contributes to delayed wound closure in apolipoprotein E knockout mice. Cell Death Differ. 2013;20:1404–14.
Hendel A, Granville DJ. Granzyme B cleavage of fibronectin disrupts endothelial cell adhesion, migration and capillary tube formation. Matrix Biol. 2013;32:14–22.
Shen Y, et al. Granzyme B deficiency protects against angiotensin II–induced cardiac fibrosis. Am J Pathol. 2016;186:87–100.
Bayer AL, et al. Cytotoxic T cells drive doxorubicin-induced cardiac fibrosis and systolic dysfunction. Nat Cardiovasc Res. 2024;3:970–86.
Kaye J. CD160 and BTLA: LIGHTs out for CD4+ T cells. Nat Immunol. 2008;9:122–4.
Lai C, et al. CD8+CD103+ tissue-resident memory T cells convey reduced protective immunity in cutaneous squamous cell carcinoma. J Immunother Cancer. 2021;9:e001807.
Tan CL, et al. CD160 stimulates CD8+ T-cell responses and is required for optimal protective immunity to Listeria monocytogenes. ImmunoHorizons. 2018;2:238–50.
Liu S, Zhang W, Liu K, Wang Y. CD160 expression on CD8+ T cells is associated with active effector responses but limited activation potential in pancreatic cancer. Cancer Immunol Immunother. 2020;69:789–97.
Rodriguez-Barbosa JI, et al. HVEM, a cosignaling molecular switch, and its interactions with BTLA, CD160 and LIGHT. Cell Mol Immunol. 2019;16:679–82.
Zhao M, et al. Targeting fibrosis: mechanisms and clinical trials. Signal Transduct Target Ther. 2022;7:206.
Zhao X, Chen J, Sun H, Zhang Y, Zou D. New insights into fibrosis from the ECM degradation perspective: the macrophage-MMP-ECM interaction. Cell Biosci. 2022;12:117.
Liu B-q, et al. XAF1 prevents hyperproduction of type I interferon upon viral infection by targeting IRF7. EMBO Rep. 2023;24:e55387.
Trapnell C, et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol. 2014;32:381–6.
Acknowledgements
We thank the Life Sciences Institute core facilities, Zhejiang University, for their technical assistance. This study was also supported by the National Key Research and Development Program of China (2025YFA1309100), the distinguished Young Scientist Fund of NSFC (82125016), and the National Natural Science Foundation of China Key Program (82230061). This research was supported by the National Natural Science Foundation of China, Special Program (82341216), and the Zhejiang Provincial Natural Science Foundation of China (LHDMD22H100002). This study was supported by the National Key Research and Development Program of China (2021YFA1101803 and 2021ZD0203304). This study was also supported by the Jiangsu Science and Technology Project (Social Development) (BE2019669) and the National Natural Science Foundation of China (82071046, 82100540). This study was also supported by the 111 Program (D20036).
Author information
Authors and Affiliations
Contributions
Conceptualization, JJ and Y-yL; methodology, KS, J-xD, J-hX, YW, X-mT, D-dL, J-yZ, M-lT, and W-pL; investigation, KS, J-xD, YW, J-hX, X-mT, D-dL, J-yZ and M-lT; investigation, KS, J-xD, YW, X-mT, D-dL, J-yZ and M-lT; writing-original draft, JJ, Y-yL, KS and J-xD; writing-review & editing, JJ, and Y-yL; visualization, JJ, and Y-yL; supervision, JJ, and Y-yL; funding acquisition, JJ, and Y-yL.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, K., Dong, Jx., Mao, Xt. et al. CD160+ intraepithelial lymphocytes and CCRL2+ macrophages drive differential repair in cardiac and liver injuries. Cell Mol Immunol 23, 186–203 (2026). https://doi.org/10.1038/s41423-025-01376-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41423-025-01376-6