Abstract
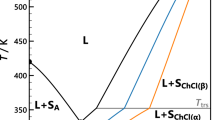
Ionic complexes consisting of a mesogenic cholesterol derivative and 1-alkyl (Cn)-3-methylimidazolium (CnMim) (n = 6–18) were prepared from ethanol solutions containing an equimolar mixture of cholesterol hydrogen phthalate (CHP) and 1-Cn-3-methylimidazolium hydroxide; the imidazolium hydroxide was obtained by anion exchange of 1-Cn-3-methylimidazolium bromide. The complex samples, termed [CnMim][CHP], were examined to evaluate their thermal transition patterns. Excluding the two samples (n = 6, 8) that showed no definite ordered phase, the complexes with n ≥ 10 formed a cholesteric (n = 10, 12) or smectic (n = 14–18) mesophase in a considerably wide range of temperatures; this wide range reflects the additional thermotropic property of the salts of CnMim with longer alkyl chains. These fluid mesophases transformed into a mesomorphic vitreous solid without crystallization in a usual cooling process. For the glassy mesomorphic samples of selected complexes (n = 10, 18), the enthalpy relaxation behavior was followed as a function of the aging temperature and time, and the data were analyzed in terms of a Kohlrausch–Williams–Watts (KWW) type of stretched exponential equation. A very narrow distribution of relaxation times was observed for the “liquid crystalline glasses”, indicating the high uniformity of the relaxation mode.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Tsuji K, Sorai M, Seki S. New finding of glassy liquid crystal—a non-equilibrium state of cholesteryl hydrogen phthalate. Bull Chem Soc Jpn. 1971;44:1452–1452.
Wedler W, Demus D, Zaschke H, Mohr K, Schäfer W, Weissflog W. Vitrification in low-molecular-weight mesogenic compounds. J Mater Chem. 1991;1:347–56.
Tamaoki N. Cholesteric liquid crystals for color information technology. Adv Mater. 2001;13:1135–47.
Chen SH, Shi H, Conger BM, Mastrangelo JC, Tsutsui T. Novel vitrifiable liquid crystals as optical materials. Adv Mater. 1996;8:998–1001.
Fan FY, Culligan SW, Mastrangelo JC, Katsis D, Chen SH, Blanton TN. Novel glass-forming liquid crystals. 6. High-temperature glassy nematics. Chem Mater. 2001;13:4584–94.
Van De Witte P, Lub J. Optical components from a new vitrifying liquid crystal. Liq Cryst. 1999;26:1039–46.
Tamaoki N, Aoki Y, Moriyama M, Kidowaki M. Photochemical phase transition and molecular realignment of glass-forming liquid crystals containing cholesterol/azobenzene dimesogenic compounds. Chem Mater. 2003;15:719–26.
Kimura N, Takeshima N, Nishio Y, Suzuki H. Phase behavior of novel liquid-crystalline salts containing a cholesteryl group. Mol Cryst Liq Cryst. 1996;287:35–45.
Yoshio M, Miyashita Y, Nishio Y. Enthalpy relaxation behavior of liquid-crystalline glasses of an esterified cholesterol derivative and its complex salts with aliphatic amines. Mol Cryst Liq Cryst. 2001;357:27–42.
Nishio Y, Chiba R. Structural characteristics and novel functionalisation of liquid-crystalline polysaccharides and cholesterol derivatives. Ekisho. 2003;7:218–27.
Welton T. Room-temperature ionic liquids: solvents for synthesis and catalysis. Chem Rev. 1999;99:2071–83.
Hallett JP, Welton T. Room-temperature ionic liquids: solvents for synthesis and catalysis. 2. Chem Rev. 2011;111:3508–76.
Armand M, Endres F, MacFarlane DR, Ohno H, Scrosati B. Ionic-liquid materials for the electrochemical challenges of the future. Nat Mater. 2009;8:621–9.
Binnemans K. Ionic liquid crystals. Chem Rev. 2005;105:4148–204.
Axenov KV, Laschat S. Thermotropic ionic liquid crystals. Materials. 2011;4:206–59.
Goossens K, Lava K, Bielawski CW, Binnemans K. Ionic liquid crystals: versatile materials. Chem Rev. 2016;116:4643–807.
Yoshio M, Mukai T, Ohno H, Kato T. One-dimensional ion transport in self-organized columnar ionic liquids. J Am Chem Soc. 2004;126:994–5.
Shimura H, Yoshio M, Hoshino K, Mukai T, Ohno H, Kato T. Noncovalent approach to one-dimensional ion conductors: enhancement of ionic conductivities in nanostructured columnar liquid crystals. J Am Chem Soc. 2008;130:1759–65.
Bonhôte P, Dias A-P, Papageorgiou N, Kalyanasundaram K, Grätzel M. Hydrophobic, highly conductive ambient-temperature molten salts. Inorg Chem. 1996;35:1168–78.
Huddleston JG, Visser AE, Reichert WM, Willauer HD, Broker GA, Rogers RD. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001;3:156–64.
Fukumoto K, Yoshizawa M, Ohno H. Room temperature ionic liquids from 20 natural amino acids. J Am Chem Soc. 2005;127:2398–9.
Richardson MJ, Savill NG. Derivation of accurate glass transition temperatures by differential scanning calorimetry. Polymer (Guildf). 1975;16:753–7.
Kimura N, Aizawa K, Nishio Y, Suzuki H. Determination of glass transition temperature by differential scanning calorimetry. Kobunshi Ronbunshu. 1996;53:866–8.
Vitz J, Erdmenger T, Haensch C, Schubert US. Extended dissolution studies of cellulose in imidazolium based ionic liquids. Green Chem. 2009;11:417–24.
Matthews RP, Villar-Garcia IJ, Weber CC, Griffith J, Cameron F, Hallett JP, Hunt PA, Welton T. A structural investigation of ionic liquid mixtures. Phys Chem Chem Phys. 2016;18:8608–24.
Bowlas CJ, Bruce DW, Seddon KR. Liquid-crystalline ionic liquids. Chem. Commun. 1996; 1625–6.
Bradley AE, Hardacre C, Holbrey JD, Johnston S, McMath SEJ, Nieuwenhuyzen. Small-angle X-ray scattering studies of liquid crystalline 1-alkyl-3-methylimidazolium salts. Chem Mater. 2002;14:629–35.
Getsis A, Mudring A-V. Imidazolium based ionic liquid crystals: structure, photophysical and thermal behaviour of [Cnmim]Br·xH2O (n=12, 14; x=0, 1). Cryst Res Technol. 2008;43:1187–96.
Weitz A, Wunderlich B. Thermal analysis and dilatometry of glasses formed under elevated pressure. J Polym Sci Polym Phys Ed. 1974;12:2473–91.
Cowie JMG, Ferguson R. Physical aging studies in poly(vinyl methyl ether). 1. Enthalpy Relaxation as a function of aging temperature. Macromolecules. 1989;22:2307–12.
Mininni RM, Moore RS, Flick JR, Petrie SEB. The effect of excess volume on molecular mobility and on the mode of failure of glassy poly(ethylene terephthalate). J Macromol Sci Part B. 1973;8:343–59.
Cavaille JY, Etienne S, Perez J, Monnerie L, Johari GP. Dynamic shear measurements of physical ageing and the memory effect in a polymer glass. Polymer (Guildf). 1986;27:686–92.
Bauwens-Crowet C, Bauwens J-C. Annealing of polycarbonate below the glass transition temperature up to equilibrium: a quantitative interpretation of enthalpy relaxation. Polymer (Guildf). 1986;27:709–13.
Struik LCE. Physical aging in amorphous polymers and other materials. Amsterdam, Netherland: Elsevier Scientific Pub. Co.; 1978.
Williams G, Watts DC. Non-symmetrical dielectric relaxation behaviour arising from a simple empirical decay function. Trans Faraday Soc. 1970;66:80–85.
Angell CA. Relaxation in liquids, polymers and plastic crystals—strong/fragile patterns and problems. J Non Cryst Solids. 1991;131-133:13–31.
Yoshida H. Enthalpy relaxation and fragility of amorphous polymers. Kobunshi Ronbunshu. 1996;53:874–6.
Yoshida H. Enthalpy relaxation of polymeric glasses. Netsu Sokutei. 1986;13:191–9.
Tanaka Y, Udagawa H. Structural relaxation of a side-chain type liquid crystalline polymer having longer spacer chain: analysis of enthalpy relaxation with an activation energy specrum. Kobunshi Ronbunshu. 2009;66:463–9.
Böhmer R. Non-linearity and non-exponentiality of primary relaxations. J Non Cryst Solids. 1994;172–174:628–34.
Tokita M, Funaoka S, Watanabe J. Study on smectic liquid crystal glass and isotropic liquid glass formed by thermotropic main-chain liquid crystal polyester. Macromolecules. 2004;37:9916–21.
Acknowledgements
This work was partially financed by a Grant-in-Aid for Scientific Research (A) (No. 26252025 to YN) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Nakajima, I., Kitaguchi, T., Sugimura, K. et al. Mesomorphic glass-forming ionic complexes composed of a cholesterol phthalate and 1-Cn-3-methylimidazolium: phase transition and enthalpy relaxation behavior. Polym J 50, 899–909 (2018). https://doi.org/10.1038/s41428-018-0047-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41428-018-0047-5