Abstract
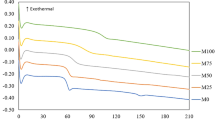
Poly(oligo(ethylene glycol) methyl ether methacrylate) (POEGMA) is known as an ionic conductive polymer and exhibits ionic conductivity when it forms a complex with a metal salt. In this study, poly(L-lactic acid) (PLLA)/POEGMA/poly(methyl methacrylate) (PMMA) blends were manufactured to develop high-performance polymer materials that exhibit high mechanical moduli (>1 GPa) and ionic conductivities in the semiconductive region (1.0 × 10 −9–1.0 × 10−7 cm−1) at room temperature. In addition, crystallized blends were prepared by annealing the amorphous blends and were subsequently evaluated. By mixing PLLA and the compound consisting of POEGMA and PMMA using a two-roll mill, a blend with a maximum ionic conductivity of ~1.1 × 10–7 S cm−1 was obtained. The scanning electron microscopy results indicated that the ionic conductive phases were finely dispersed within the blends. By comparing the storage modulus values of the crystallized blends to those of amorphous blends, we found that the crystallized blends were harder at approximately the glass transition temperature of PLLA.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Tarascon J-M, Armand M. Issues and challenges facing rechargeable lithium batteries. Nature. 2001;414:359–67.
Armand M, Tarascon J-M. Building better batteries. Nature. 2008;451:652–7.
Scrosati B, Hassoun J, Sun Y-K. Lithium-ion batteries. A look into the future. Energy Environ Sci. 2011;4:3287–95.
Nogueira AF, Durrant JR, De Paoli MA. Dye-sensitized nanocrystalline solar cells employing a polymer electrolyte. Adv Mater. 2001;13:826–30.
Wang P, Zakeeruddin SM, Moser JE, Nazeeruddin MK, Sekiguchi T, Gratzel M. A stable quasi-solid-state dye-sensitized solar cell with an amphiphilic ruthenium sensitizer and polymer gel electrolyte. Nat Mater. 2003;2:402–7.
Fenton DE, Parker JM, Wright PV. Complexes of alkali metal ions with poly(ethylene oxide). Polymer. 1973;14:589.
Armand MB, Chabagno JM, Duclot MT. Fast ion transport in solids. New York: Elsevier North Holland; 1979. p. 131.
Meyer WH. Polymer electrolytes for lithium-ion batteries. Adv Mater. 1998;10:439–48.
Xia DW, Soltz D, Smid J. Conductivities of solid polymer electrolyte complexes of alkali salts with polymers of methoxypolyethyleneglycol methacrylates. Solid State Ion. 1984;14:221–4.
Kobayashi N, Uchiyama M, Shigehara K, Tsuchida E. Ionically high conductive solid electrolytes composed of graft copolymer-lithium salt hybrids. J Phys Chem. 1985;89:987–91.
Ding L, Shi J, Yang C, Dong S. Ionic conductivity of solid polymer electrolytes based on modified alternating maleic anhydride copolymer with oligo(oxyethylene) side chains. Polym J. 1997;29:410–6.
Keld W, Birgit ZC, Torben J, Emil HL, Steen S. Poly(ethylene oxide)-sodium perchlorate electrolytes in solid-state sodium cells. Br Polym J. 1988;20:243–6.
Kobayashi Y. Exploitation technology and application of electroconductive nano filler. Tokyo: Shii Emu Shii shuppan; 2005.
Hou L, Yang G. Morphology and thermal properties of MCPA6/ABS by in situ polymerization of ε-caprolactam. Macromol Chem Phys. 2005;206:1887–95.
Guo Q, Arends P, Thomann R, Spontak RJ, Gronski W. Morphological development and rheological changes of phenoxy/SAN blends during in-situ polymerization. J Polym Sci B. 2007;45:2614–9.
Cai H, Dave V, Gross RA, McCarthy SP. Effects of crystallinity and orientation on the enzymatic degradation of poly(lactic acid). ANTEC’. 1995;95:2046–50.
Song DK, Sung YK. Synthesis and characterization of biodegradable poly(1,4‐butanediol succinate). J Appl Polym Sci. 1995;56:1381–95.
Wendl B, Droschl H, Kern W. A comparative study of polymerization lamps to determine the degree of cure of composites using infrared spectroscopy. Eur J Orthod. 2004;26:545–51.
Hyo-Jeong H, Eun-Hye K, Yo HK, Chang JYK, Sang-Young KL, UV-curable L. semi-interpenetrating polymer network-integrated, highly bendable plastic crystal composite electrolytes for shape-confortable all-solid-state lithium ion batteries. Energy Environ Sci. 2012;5:6491–9.
Pan P, Kai W, Zhu B, Dong T, Inoue Y. Polymorphous crystallization and multiple melting behavior of poly(l-lactide): molecular weight dependence. Macromolecules. 2007;40:6898–905.
Kawai T, Rahman N, Matsuba G, Nishida K, Kanaya T, Nakano M. et al. Crystallization and melting behavior of poly (l-lactic acid). Macromolecules. 2007;40:9463–9.
Fischer EW, Sterzel HJ, Wegner GK. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid Z Z Polym. 1973;251:980–90.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yamada, R., Inoue, I., Akasaka, S. et al. Phase structure and electrical and mechanical properties of PLLA/ionic conductive polyether blends prepared by melt mixing. Polym J 51, 649–656 (2019). https://doi.org/10.1038/s41428-019-0176-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41428-019-0176-5