Abstract
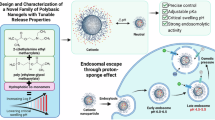
Various polymeric nanoparticles have been used as drug carriers in drug delivery systems (DDSs). Most of them were constructed from dynamic self-assembly systems formed via hydrophobic interactions and from structures that are unstable in an in vivo environment owing to their relatively weak formation forces. As a solution to this issue, physically stabilized core-crosslinked particles (CP) with chemically crosslinked cores have received attention as alternatives to the dynamic nanoparticles. This focused review summarizes recent advances in the construction, structural characterization, and in vivo behavior of polymeric CPs. First, we introduce a nanoemulsion-mediated method to create polyethylene glycol (PEG)-bearing CPs and their structural characterization. The relationship between the PEG chain conformations in the particle shell and the in vivo fate of the CPs is also discussed. After that, the development and advantages of zwitterionic amino acid-based polymer (ZAP)-bearing CPs are presented to address the poor penetration and the internalization of PEG-based CPs into tumor tissues and cells, respectively. Finally, we conclude and discuss prospects for application of polymeric CPs in the DDS field.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
van Rijt SH, Sadler PJ. Current applications and future potential for bioinorganic chemistry in the development of anticancer drugs. Drug Discov Today. 2009;14:1089–97. https://doi.org/10.1016/j.drudis.2009.09.003
Ghosh S. Cisplatin: The first metal based anticancer drug. Bioorg Chem. 2019;88:102925 https://doi.org/10.1016/j.bioorg.2019.102925
Heishima K, Sugito N, Soga T, Nishikawa M, Ito Y, Honda R, et al. Petasin potently inhibits mitochondrial complex I–based metabolism that supports tumor growth and metastasis. J Clin Investig. 2021;131:e139933 https://doi.org/10.1172/JCI139933
Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, Santos MS, et al. Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem. 2009;16:3267–85. https://doi.org/10.2174/092986709788803312.
Saraf S, Jain A, Tiwari A, Verma A, Panda PK, Jain SK. Advances in liposomal drug delivery to cancer: An overview. J Drug Delivery Sci Technol. 2020;56:101549 https://doi.org/10.1016/j.jddst.2020.101549
Lee JS, Feijen J. Polymersomes for drug delivery: Design, formation and characterization. J Contr Release. 2012;161:473–83. https://doi.org/10.1016/j.jconrel.2011.10.005
Liu Y, Castro Bravo KM, Liu J. Targeted liposomal drug delivery: a nanoscience and biophysical perspective. Nanoscale Horizons. 2021;6:78–94. https://doi.org/10.1039/D0NH00605J
Ghezzi M, Pescina S, Padula C, Santi P, Del Favero E, Cantù L, et al. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J Contr Release. 2021;332:312–36. https://doi.org/10.1016/j.jconrel.2021.02.031
Hwang D, Ramsey JD, Kabanov AV. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv Drug Delivery Rev. 2020;156:80–118. https://doi.org/10.1016/j.addr.2020.09.009
Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharmacy Pharmacol. 2012;65:157–70. https://doi.org/10.1111/j.2042-7158.2012.01567.x. (acccessed 1/20/2023)
Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv Drug Delivery Rev. 2012;64:37–48. https://doi.org/10.1016/j.addr.2012.09.013
Tanner P, Baumann P, Enea R, Onaca O, Palivan C, Meier W. Polymeric Vesicles: From Drug Carriers to Nanoreactors and Artificial Organelles. Acc Chem Res. 2011;44:1039–49. https://doi.org/10.1021/ar200036k
Nishimura T, Akiyoshi K. Biotransporting Biocatalytic Reactors toward Therapeutic Nanofactories. Adv Sci. 2018;5:1800801 https://doi.org/10.1002/advs.201800801
Yue J, Liu S, Xie Z, Xing Y, Jing X. Size-dependent biodistribution and antitumor efficacy of polymer micelle drug delivery systems. J Mater Chem B. 2013;1:4273–80. https://doi.org/10.1039/C3TB20296H
Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–92.
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Controlled Release. 2000;65:271–84. https://doi.org/10.1016/S0168-3659(99)00248-5
Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6:815–23. https://doi.org/10.1038/nnano.2011.166
Sindhwani S, Syed AM, Ngai J, Kingston BR, Maiorino L, Rothschild J, et al. The entry of nanoparticles into solid tumours. Nat Mater. 2020;19:566–75. https://doi.org/10.1038/s41563-019-0566-2
Kingston BR, Lin ZP, Ouyang B, MacMillan P, Ngai J, Syed AM, et al. Specific Endothelial Cells Govern Nanoparticle Entry into Solid Tumors. ACS Nano. 2021;15:14080–94. https://doi.org/10.1021/acsnano.1c04510
Matsumura Y. Preclinical and clinical studies of NK012, an SN-38-incorporating polymeric micelles, which is designed based on EPR effect. Adv Drug Delivery Rev. 2011;63:184–92. https://doi.org/10.1016/j.addr.2010.05.008
Maeda H. The 35th Anniversary of the Discovery of EPR Effect: A New Wave of Nanomedicines for Tumor-Targeted Drug Delivery—Personal Remarks and Future Prospects. J Personalized Med. 2021;11:229.
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–51. https://doi.org/10.1038/nbt.3330
D’souza AA, Shegokar R. Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Exp Opin Drug Delivery. 2016;13:1257–75. https://doi.org/10.1080/17425247.2016.1182485
Matsumura Y. 35 years of discussions with Prof. Maeda on the EPR effect and future directions. J Controlled Release. 2022;348:966–9. https://doi.org/10.1016/j.jconrel.2022.06.035
Shi D, Beasock D, Fessler A, Szebeni J, Ljubimova JY, Afonin KA, et al. To PEGylate or not to PEGylate: Immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv Drug Delivery Rev. 2022;180:114079 https://doi.org/10.1016/j.addr.2021.114079
Fujii S, Sakuragi M, Sakurai K. Characterizing PEG. Chains Tethered onto Micelles and Liposomes Applied as Drug Delivery Vehicles Using Scattering Techniques. Control of Amphiphile Self-Assembling at the Molecular Level: Supra-Molecular Assemblies with Tuned Physicochemical Properties for Delivery Applications, ACS Symposium Series. 1271. American Chemical Society; 2017. p. 115–29. https://doi.org/10.1021/bk-2017-1271.ch005.
Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–60. https://doi.org/10.1038/nrd1632
Klimek L, Novak N, Cabanillas B, Jutel M, Bousquet J, Akdis CA. Allergenic components of the mRNA-1273 vaccine for COVID-19: Possible involvement of polyethylene glycol and IgG-mediated complement activation. Allergy. 2021;76:3307–13. https://doi.org/10.1111/all.14794
Ouyang B, Poon W, Zhang Y-N, Lin ZP, Kingston BR, Tavares AJ, et al. The dose threshold for nanoparticle tumour delivery. Nat Mater. 2020;19:1362–71. https://doi.org/10.1038/s41563-020-0755-z
Liu J, Zeng F, Allen C. In vivo fate of unimers and micelles of a poly(ethylene glycol)-block-poly(caprolactone) copolymer in mice following intravenous administration. Eur J Pharma Biopharma. 2007;65:309–19. https://doi.org/10.1016/j.ejpb.2006.11.010
Ebrahim Attia AB, Yang C, Tan JPK, Gao S, Williams DF, Hedrick JL, et al. The effect of kinetic stability on biodistribution and anti-tumor efficacy of drug-loaded biodegradable polymeric micelles. Biomaterials. 2013;34:3132–40. https://doi.org/10.1016/j.biomaterials.2013.01.042
Wang Y, Pisapati AV, Zhang XF, Cheng X. Recent Developments in Nanomaterial-Based Shear-Sensitive Drug Delivery Systems. Adv Healthcare Mater. 2021;10:2002196 https://doi.org/10.1002/adhm.202002196
Owen SC, Chan DPY, Shoichet MS. Polymeric micelle stability. Nano Today. 2012;7:53–65. https://doi.org/10.1016/j.nantod.2012.01.002
Feiner-Gracia N, Glinkowska Mares A, Buzhor M, Rodriguez-Trujillo R, Samitier Marti J, Amir RJ, et al. Real-Time Ratiometric Imaging of Micelles Assembly State in a Microfluidic Cancer-on-a-Chip. ACS Appl Bio Mater. 2021;4:669–81. https://doi.org/10.1021/acsabm.0c01209
Sun X, Wang G, Zhang H, Hu S, Liu X, Tang J, et al. The Blood Clearance Kinetics and Pathway of Polymeric Micelles in Cancer Drug Delivery. ACS Nano. 2018;12:6179–92. https://doi.org/10.1021/acsnano.8b02830
O’Reilly RK, Hawker CJ, Wooley KL. Cross-linked block copolymer micelles: functional nanostructures of great potential and versatility. Chem Soc Rev. 2006;35:1068–83. https://doi.org/10.1039/B514858H
Liao C, Chen Y, Yao Y, Zhang S, Gu Z, Yu X. Cross-Linked Small-Molecule Micelle-Based Drug Delivery System: Concept, Synthesis, and Biological Evaluation. Chem Mater. 2016;28:7757–64. https://doi.org/10.1021/acs.chemmater.6b02965
Yoo D, Magsam AW, Kelly AM, Stayton PS, Kievit FM, Convertine AJ. Core-Cross-Linked Nanoparticles Reduce Neuroinflammation and Improve Outcome in a Mouse Model of Traumatic Brain Injury. ACS Nano. 2017;11:8600–11. https://doi.org/10.1021/acsnano.7b03426
Gebrie HT, Addisu KD, Darge HF, Birhan YS, Thankachan D, Tsai H-C, et al. pH/redox-responsive core cross-linked based prodrug micelle for enhancing micellar stability and controlling delivery of chemo drugs: An effective combination drug delivery platform for cancer therapy. Biomater Adv. 2022;139:213015 https://doi.org/10.1016/j.bioadv.2022.213015
Parent LR, Bakalis E, Ramírez-Hernández A, Kammeyer JK, Park C, de Pablo J, et al. Directly Observing Micelle Fusion and Growth in Solution by Liquid-Cell Transmission Electron Microscopy. J Am Chem Soc. 2017;139:17140–51. https://doi.org/10.1021/jacs.7b09060
Tian Q, Fei C, Yin H, Feng Y. Stimuli-responsive polymer wormlike micelles. Prog Polymer Sci. 2019;89:108–32. https://doi.org/10.1016/j.progpolymsci.2018.10.001
Lund R, Willner L, Monkenbusch M, Panine P, Narayanan T, Colmenero J, et al. Structural Observation and Kinetic Pathway in the Formation of Polymeric Micelles. Phys Rev Lett. 2009;102:188301 https://doi.org/10.1103/PhysRevLett.102.188301
Lund R, Brun G, Chevallier E, Narayanan T, Tribet C. Kinetics of Photocontrollable Micelles: Light-Induced Self-Assembly and Disassembly of Azobenzene-Based Surfactants Revealed by TR-SAXS. Langmuir. 2016;32:2539–48. https://doi.org/10.1021/acs.langmuir.5b04711
Iijima M, Nagasaki Y, Okada T, Kato M, Kataoka K. Core-Polymerized Reactive Micelles from Heterotelechelic Amphiphilic Block Copolymers. Macromolecules. 1999;32:1140–6. https://doi.org/10.1021/ma9815962
Bontha S, Kabanov AV, Bronich TK. Polymer micelles with cross-linked ionic cores for delivery of anticancer drugs. J Controlled Release. 2006;114:163–74. https://doi.org/10.1016/j.jconrel.2006.06.015
Rijcken CJ, Snel CJ, Schiffelers RM, van Nostrum CF, Hennink WE. Hydrolysable core-crosslinked thermosensitive polymeric micelles: Synthesis, characterisation and in vivo studies. Biomaterials. 2007;28:5581–93. https://doi.org/10.1016/j.biomaterials.2007.08.047
Tanaka R, Arai K, Matsuno J, Soejima M, Lee JH, Takahashi R, et al. Furry nanoparticles: synthesis and characterization of nanoemulsion-mediated core crosslinked nanoparticles and their robust stability in vivo. Polymer Chem. 2020;11:4408–16. https://doi.org/10.1039/D0PY00610F
Matsuno J, Kanamaru T, Arai K, Tanaka R, Lee JH, Takahashi R, et al. Synthesis and characterization of nanoemulsion-mediated core crosslinked nanoparticles, and in vivo pharmacokinetics depending on the structural characteristics. J Controlled Release. 2020;324:405–12. https://doi.org/10.1016/j.jconrel.2020.05.035
Zheng P, McCarthy TJ. D4H/D4V Silicone: A Replica Material with Several Advantages for Nanoimprint Lithography and Capillary Force Lithography. Langmuir. 2011;27:7976–9. https://doi.org/10.1021/la201141k
Fujii S, Yamada S, Matsumoto S, Kubo G, Yoshida K, Tabata E, et al. Platonic Micelles: Monodisperse Micelles with Discrete Aggregation Numbers Corresponding to Regular Polyhedra. Sci Rep. 2017;7:44494 https://doi.org/10.1038/srep44494
Klein M, Menta M, Dacoba TG, Crecente-Campo J, Alonso MJ, Dupin D, et al. Advanced nanomedicine characterization by DLS and AF4-UV-MALS: Application to a HIV nanovaccine. J Pharma Biomed Analy. 2020;179:113017 https://doi.org/10.1016/j.jpba.2019.113017
Écija-Arenas Á, Román-Pizarro V, Fernández-Romero JM. Separation and characterization of liposomes using asymmetric flow field-flow fractionation with online multi-angle light scattering detection. J Chromatogr A. 2021;1636:461798 https://doi.org/10.1016/j.chroma.2020.461798
Yang Q, Jones SW, Parker CL, Zamboni WC, Bear JE, Lai SK. Evading Immune Cell Uptake and Clearance Requires PEG Grafting at Densities Substantially Exceeding the Minimum for Brush Conformation. Mol Pharma. 2014;11:1250–8. https://doi.org/10.1021/mp400703d
Du X-J, Wang J-L, Liu W-W, Yang J-X, Sun C-Y, Sun R, et al. Regulating the surface poly(ethylene glycol) density of polymeric nanoparticles and evaluating its role in drug delivery in vivo. Biomaterials. 2015;69:1–11. https://doi.org/10.1016/j.biomaterials.2015.07.048
Zhao Z, Ukidve A, Krishnan V, Mitragotri S. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv Drug Delivery Rev. 2019;143:3–21. https://doi.org/10.1016/j.addr.2019.01.002
Cao Z-T, Gan L-Q, Jiang W, Wang J-L, Zhang H-B, Zhang Y, et al. Protein Binding Affinity of Polymeric Nanoparticles as a Direct Indicator of Their Pharmacokinetics. ACS Nano. 2020;14:3563–75. https://doi.org/10.1021/acsnano.9b10015
Wang J-L, Du X-J, Yang J-X, Shen S, Li H-J, Luo Y-L, et al. The effect of surface poly(ethylene glycol) length on in vivo drug delivery behaviors of polymeric nanoparticles. Biomaterials. 2018;182:104–13. https://doi.org/10.1016/j.biomaterials.2018.08.022
Perry JL, Reuter KG, Kai MP, Herlihy KP, Jones SW, Luft JC, et al. PEGylated PRINT Nanoparticles: The Impact of PEG Density on Protein Binding, Macrophage Association, Biodistribution, and Pharmacokinetics. Nano Lett. 2012;12:5304–10. https://doi.org/10.1021/nl302638g
Kanamaru T, Sakurai K, Fujii S. Impact of Polyethylene Glycol (PEG) Conformations on the In Vivo Fate and Drug Release Behavior of PEGylated Core-Cross-Linked Polymeric Nanoparticles. Biomacromolecules. 2022;23:3909–18. https://doi.org/10.1021/acs.biomac.2c00730
Nagarajan R, Ruckenstein E. Theory of surfactant self-assembly: a predictive molecular thermodynamic approach. Langmuir. 1991;7:2934–69. https://doi.org/10.1021/la00060a012
Li Y, Xiao K, Luo J, Xiao W, Lee JS, Gonik AM, et al. Well-defined, reversible disulfide cross-linked micelles for on-demand paclitaxel delivery. Biomaterials. 2011;32:6633–45. https://doi.org/10.1016/j.biomaterials.2011.05.050
Zhao J, Yan C, Chen Z, Liu J, Song H, Wang W, et al. Dual-targeting nanoparticles with core-crosslinked and pH/redox-bioresponsive properties for enhanced intracellular drug delivery. J Coll Interfac Sci. 2019;540:66–77. https://doi.org/10.1016/j.jcis.2019.01.021
Talelli M, Barz M, Rijcken CJ, Kiessling F, Hennink WE, Lammers T. Core-Crosslinked Polymeric Micelles: Principles, Preparation, Biomedical Applications and Clinical Translation. Nano Today. 2015;10:93–117. https://doi.org/10.1016/j.nantod.2015.01.005. From NLM
Zalba S, ten Hagen TLM, Burgui C, Garrido MJ. Stealth nanoparticles in oncology: Facing the PEG dilemma. J Controlled Release. 2022;351:22–36. https://doi.org/10.1016/j.jconrel.2022.09.002
Miura Y, Hoshino Y, Seto H. Glycopolymer Nanobiotechnology. Chem Rev. 2016;116:1673–92. https://doi.org/10.1021/acs.chemrev.5b00247
Zhong Y, Meng F, Deng C, Zhong Z. Ligand-Directed Active Tumor-Targeting Polymeric Nanoparticles for Cancer Chemotherapy. Biomacromolecules. 2014;15:1955–69. https://doi.org/10.1021/bm5003009
Zununi Vahed S, Fathi N, Samiei M, Maleki Dizaj S, Sharifi S. Targeted cancer drug delivery with aptamer-functionalized polymeric nanoparticles. J Drug Targeting. 2019;27:292–9. https://doi.org/10.1080/1061186X.2018.1491978
Li W, Liu Q, Liu L. Antifouling Gold Surfaces Grafted with Aspartic Acid and Glutamic Acid Based Zwitterionic Polymer Brushes. Langmuir. 2014;30:12619–26. https://doi.org/10.1021/la502789v
Alswieleh AM, Cheng N, Canton I, Ustbas B, Xue X, Ladmiral V, et al. Zwitterionic Poly(amino acid methacrylate) Brushes. J Am Chem Soc. 2014;136:9404–13. https://doi.org/10.1021/ja503400r
Fuchs BC, Bode BP. Amino acid transporters ASCT2 and LAT1 in cancer: Partners in crime. Semin Cancer Biol. 2005;15:254–66. https://doi.org/10.1016/j.semcancer.2005.04.005
Häfliger P, Charles R-P. The L-Type Amino Acid Transporter LAT1—An Emerging Target in Cancer. Int J Mol Sci. 2019;20:2428.
Yamada N, Honda Y, Takemoto H, Nomoto T, Matsui M, Tomoda K, et al. Engineering Tumour Cell-Binding Synthetic Polymers with Sensing Dense Transporters Associated with Aberrant Glutamine Metabolism. Sc Rep. 2017;7:6077 https://doi.org/10.1038/s41598-017-06438-y
Takano S, Sakurai K, Fujii S. Internalization into cancer cells of zwitterionic amino acid polymers via amino acid transporter recognition. Polym Chem. 2021;12:6083–7. https://doi.org/10.1039/D1PY01010G
Leiske MN, Mazrad ZAI, Zelcak A, Wahi K, Davis TP, McCarroll JA, et al. Zwitterionic Amino Acid-Derived Polyacrylates as Smart Materials Exhibiting Cellular Specificity and Therapeutic Activity. Biomacromolecules. 2022;23:2374–87. https://doi.org/10.1021/acs.biomac.2c00143
Fujii S, Sakurai K. Zwitterionic Amino Acid Polymer-Grafted Core-Crosslinked Particle toward Tumor Delivery. Biomacromolecules. 2022;23:3968–77. https://doi.org/10.1021/acs.biomac.2c00803
Zhao L-P, Chen S-Y, Zheng R-R, Kong R-J, Rao X-N, Chen AL, et al. Self-Delivery Nanomedicine for Glutamine-Starvation Enhanced Photodynamic Tumor Therapy. Adv Healthcare Mater. 2022;11:2102038 https://doi.org/10.1002/adhm.202102038. (acccessed 2023/01/29)
Müllner M, Yang K, Kaur A, New EJ. Aspect-ratio-dependent interaction of molecular polymer brushes and multicellular tumour spheroids. Polym Chem. 2018;9:3461–5. https://doi.org/10.1039/C8PY00703A
Nakamura H, Koziolová E, Chytil P, Etrych T, Haratake M, Maeda H. Superior Penetration and Cytotoxicity of HPMA Copolymer Conjugates of Pirarubicin in Tumor Cell Spheroid. Mol Pharma. 2019;16:3452–9. https://doi.org/10.1021/acs.molpharmaceut.9b00248
Fujii S, Takano S, Nakazawa K, Sakurai K. Impact of Zwitterionic Polymers on the Tumor Permeability of Molecular Bottlebrush-Based Nanoparticles. Biomacromolecules. 2022;23:2846–55. https://doi.org/10.1021/acs.biomac.2c00216
Ozer I, Kelly G, Gu R, Li X, Zakharov N, Sirohi P, et al. Polyethylene Glycol-Like Brush Polymer Conjugate of a Protein Drug Does Not Induce an Antipolymer Immune Response and Has Enhanced Pharmacokinetics than Its Polyethylene Glycol Counterpart. Adv Sci (Weinh). 2022;9:e2103672 https://doi.org/10.1002/advs.202103672.
Acknowledgements
This work was supported by JSPS KAKENHI: Grant-in-Aid for Scientific Research (Grant Number 19K15394) and Grant-in-Aid for Scientific Research B (22H01913).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fujii, S. Polymeric core-crosslinked particles prepared via a nanoemulsion-mediated process: from particle design and structural characterization to in vivo behavior in chemotherapy. Polym J 55, 921–933 (2023). https://doi.org/10.1038/s41428-023-00793-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41428-023-00793-6
This article is cited by
-
Design of functional soft interfaces with precise control of the polymer architecture
Polymer Journal (2024)