Abstract
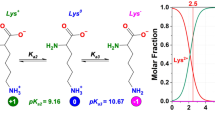
Excess copper accumulation in the body can lead to various health complications including Parkinson’s disease, Alzheimer’s disease, gastrointestinal disorders, liver damage, and hemolytic anemia. As such, the development of effective strategies to remove excess copper is critical for preventing these adverse health outcomes. In this study, a copolymer (P(MPC/LysA)) comprising 2-(methacryloyloxy)ethyl phosphorylcholine (MPC) and l-lysinylacrylamide (LysA) was synthesized via reversible addition–fragmentation chain transfer (RAFT) radical polymerization. Under different pH conditions, the pendant primary amine (–NH3+) and carboxy groups (–COOH) of LysA underwent protonation and deprotonation, resulting in cationic, zwitterionic, and anionic structures. The copolymer exhibited a zwitterionic structure under physiological conditions due to the pH-independent neutral charge of MPC. The LysA residues formed a complex with copper (II) ions (Cu2+) under neutral-basic conditions, with two pendant l-lysine residues forming a complex with one Cu2+ molecule. The addition of Cu2+ to an aqueous solution of P(MPC/LysA) at pH 7.4 resulted in the formation of interpolymer aggregates due to Cu2+/LysA complex formation. Overall, this study reveals that P(MPC/LysA) has potential for use in removing excess Cu2+ in the body by forming water-soluble aggregates with Cu2+ at physiological pH.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Tainer JA, Roberts VA, Getzoff ED. Metal-binding sites in proteins. Curr Opin Biotechnol. 1991;2:582–91.
London WP, Steck TL. Kinetics of enzyme reactions with interaction between a substrate and a (Metal) modifier. Biochemistry. 1969;8:1767–79.
Rosenzweig AC, Sazinsky MH. Structural insights into dioxygen-activating copper enzymes. Curr Opin Struct Biol. 2006;16:729–35.
Vivoli G, Bergomi M, Rovesti S, Pinotti M, Caselgrandi E. Zinc, copper, and zinc- or copper-dependant enzymes in human hypertension. Biol Trace Elem Res. 1995;49:97–106.
Cohen NL, Keen CL, Hurley LS, Lonnerdal B. Determinants of copper-deficiency anemia in rats. J Nutr. 1985;115:710–25.
Lazarchick J. Update on anemia and neutropenia in copper deficiency. Curr Opin Hematol. 2012;19:58–60.
Dollwet HHA, Sorenson JRJ. Roles of copper in bone maintenance and healing. Biol Trace Elem Res. 1988;18:39–48.
Mandelbrote BM, Stanier MW, Thompson RHS, Thruston MN. Studies on copper metabolism in demyelinating disease of the central nervous system. Brain. 1948;71:212–28.
Montes S, Rivera-Mancia S, Diaz-Ruiz A, Tristan-Lopez L, Rios C. Copper and copper proteins in Parkinson’s disease. Oxid Med Cell Longev. 2014;2014:147251.
Balland V, Hureau C, Saveant JM. Electrochemical and homogeneous electron transfers to the Alzheimer amyloid-β copper complex follow a preorganization mechanism. Proc Natl Acad Sci USA. 2010;107:17113–8.
Garcia-Nino WR, Pedraza-Chaverri J. Protective effect of curcumin against heavy metals-induced liver damage. Food Chem Toxicol. 2014;69:182–201.
Fuentealba IC, Mullins JE, Abuto EM, Lau JC, Cherian GM. Effect of age and sex on liver damage due to excess dietary copper in Fischer 344 rats. J Toxicol Clin Toxicol. 2000;38:709–17.
Ribarov SR, Benov LC. Relationship between the hemolytic action of heavy metals and lipid peroxidation. Biochim Biophys Acta. 1981;640:721–6.
Forbes JR, Hsi G, Cox DW. Role of the copper-binding domain in the copper transport function of ATP7B, the P-type ATPase defective in Wilson disease. J Biol Chem. 1999;274:12408–113.
Gu M, Cooper JM, Butler P, Walker AP, Mistry PK, Dooley JS, et al. Oxidative-phosphorylation defect in liver of patients with Wilson’s disease. Lancet 2000;356:469–74.
Purchase R. The link between copper and Wilson’s disease. Sci Prog. 2013;96:213–23.
Lorincz MT, Lang PA, Schenck M, Nicolay JP, Becker JU, Kempe DS, et al. Liver cell death and anemia in Wilson disease involve acid sphingomyelinase and ceramide. Nat Med. 2007;13:164–70.
Lorincz MT. Neurologic Wilson’s disease. Ann N Y Acad Sci. 2010;1184:173–87.
Roberts EA, Schilsky ML. Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008;47:2089–111.
Cumings JN. The effects of B. A. L. in hepatolenticular degeneration. Brain. 1951;74:10–22.
Ala A, Walker AP, Ashkan K, Dooley JS, Schisky ML. Wilson’s disease. Lancet. 2007;369:397–408.
Cao Y, Skaug MA, Anderson O, Aaseth J. Chelation therapy in intoxications with mercury, lead and copper. J Trace Elem Med Biol. 2015;31:188–92.
Weiss KH, Thurik F, Gotthardt DN, Schafer M, Teufel U, Wiegand F, et al. Efficacy and safety of oral chelators in treatment of patients with Wilson disease. Clin Gastroenterol Hepatol. 2013;11:1028–35.
Bolognin S, Drago D, Messori L, Zatta P. Chelation therapy for neurodegenerative diseases. Med Res Rev. 2009;29:547–70.
Cullen NM, Wolf LR, St Clair D. Pediatric arsenic ingestion. Am J Emerg Med. 1995;13:432–5.
Debayle M, Balloul E, Dembele F, Xu X, Hanafi M, Ribot F, et al. Zwitterionic polymer ligands: an ideal surface coating to totally suppress protein-nanoparticle corona formation? Biomaterials. 2019;219:119357.
Yin T, Chu X, Cheng J, Liang J, Zhou J, Huo M. Hypoxia-sensitive zwitterionic vehicle for tumor-specific drug delivery through antifouling-based stable biotransport alongside PDT-sensitized controlled release. Biomacromolecules. 2021;22:2233–47.
Yuan Y, Mao Y, Du X, Du J, Wang F, Wang J. Surface charge switchable nanoparticles based on zwitterionic polymer for enhanced drug delivery to tumor. Adv Mater. 2012;24:5476–80.
Cai M, Leng M, Lu A, He L, Xie X, Huang L, et al. Synthesis of amphiphilic copolymers containing zwitterionic sulfobetaine as pH and redox responsive drug carriers. Colloids Surf B. 2015;126:1–9.
Moro T, Takatori Y, Ishihara K, Konno T, Takigawa Y, Natsushita T, et al. Surface grafting of artificial joints with a biocompatible polymer for preventing periprosthetic. Nat Mater. 2004;3:829–36.
Ishihara K, Aragaki R, Ueda T, Watenabe A, Nakabayashi N. Reduced thrombogenicity of polymers having phospholipid polar groups. J Biomed Mater Res. 1990;24:1069–77.
Fujii S, Kido M, Sato M, Higaki Y, Hirai T, Ohta N. et al. pH-Responsive and selective protein adsorption on an amino acid-based zwitterionic polymer surface. Polym Chem. 2015;6:7053–9.
Ndokoye P, Ke J, Liu J, Zhao Q, Li X. L-Cysteine-modified gold nanostars for SERS-based copper ions detection in aqueous media. Langmuir. 2014;30:13491–7.
Chen PC, Lai JJ, Huang CJ. Bio-inspired amphoteric polymer for triggered-release drug delivery on breast cancer cells based on metal coordination. ACS Appl Mater Interfaces. 2021;13:25663–73.
Banerjee S, Maji T, Paira TK, Mandal TK. Amino-acid-based zwitterionic polymer and its Cu(II)-induced aggregation into nanostructures: a template for CuS and CuO nanoparticles. Macromol Rapid Commun. 2013;34:1480–6.
Kuo S, Chwn P, Huang K, Huang C. Bio-inspired zwitterionic polymeric chelating assembly for treatment of copper-induced cytotoxicity and hemolysis. Mater Sci Eng C. 2021;129:112367.
Huang KT, Hsieh PS, Dai LG, Huang CJ. Complete zwitterionic double network hydrogels with great toughness and resistance against foreign body reaction and thrombus. J Mater Chem B. 2020;8:7390–402.
Nagaoka S, Sundo A, Satoh T, Nagira K, Kishi R, Ueno K, et al. Method for a convenient and efficient synthesis of amino acid acrylic monomers with zwitterionic structure. Synth Commun. 2005;35:2529–34.
Mitsukami Y, Donovan MS, Lowe AB, McCormick CL. Water-soluble polymers. 81. Direct s ynthesis of hydrophilic styrenic-based homopolymers and block copolymers in aqueous solution via RAFT. Macromolecules. 2001;34:2248–56.
Srinivasulu B, Rao PR, Sundaram EV. Synthesis and characterization of ethyl methacrylate-acrylamide copolymers. J Appl Polym Sci. 1991;43:1521–5.
Wang Y, Zhang X, Li W, Cheng J, Liu C, Zheng J. Determination of reactivity ratios of copolymerization of acrylamide (AM) and methacryloxyethyltrimethyl ammonium chloride (DMC) with ultraviolet initiation, and their sequence length distribution. Polym Polym Compos. 2016;24:307–14.
Dinda P, Anas M, Banerjee P, Mandal TK. Dual thermoresponsive Boc-lysine-based acryl polymer: RAFT kinetics and anti-protein-fouling of its zwitterionic form. Macromolecules. 2022;55:4011–24.
Sibarani J, Takai M, Ishihara K. Surface modification on microfluidic devices with 2-methacryloyloxyethyl phosphorylcholine polymers for reducing unfavorable protein adsorption. Colloids Surf B. 2007;54:88–93.
Hidmi L, Edwards M. Role of Temperature and pH in Cu(OH)2 solubility. Environ Sci Technol. 1999;33:2607–10.
Khan MA, Meullemeestre J, Schwing MJ, Vierling F. Stability, spectra and structure of copper (II) chloride complexes in acetic acid. Polyhedron. 1983;2:459–63.
Pedersen JS. Form factors of block copolymer micelles with spherical, ellipsoidal and cylindrical cores. J Appl Cryst. 2000;33:637–40.
Beaucage G. Small-angle scattering from polymeric mass fractals of arbitrary mass-fractal dimension. J Appl Cryst. 1996;29:134–46.
Beaucage G. Approximations leading to a unified exponential/power-low approach to small-angle scattering. J Appl Cryst. 1995;28:717–28.
Kujawa P, Tanaka F, Winnik FM. Temperature-dependent properties of telechelic hydrophobically modified poly(N-isopropylacylamides) in water: evidence from light scattering and fluorescence spectroscopy for the formation of stable mesoglobules at elevated temperature. Macromolecules. 2006;39:3048–55.
Konishi T, Yoshizaki T, Yamakawa H. On the “universal constants” ρ and ϕ of flexible polymers. Macromolecules. 1991;24:5614–22.
Platten F, Hansen J, Wagner D, Egelhaaf SU. Second virial coefficient as determined from protein phase behavior. J Phys Chem Lett. 2016;7:4008–14.
Annunziata O, Payne A, Wang Y. Solubility of lysozyme in the presence of aqueous chloride salts: common-ion effect and its role on solubility and crystal thermodynamics. J Am Chem Soc. 2008;130:13347–52.
Sikora A, Shard AG, Minelli C. Size and ζ-potential measurement of silica nanoparticles in serum using tunable resistive pulse sensing. Langmuir. 2016;3:2216–24.
Multia E, Tear CJY, Palviainen M, Siljander P, Riekkola ML. Fast isolation of highly specific population of platelet-derived extracellular resicles from blood plasma by affinity monolithic column, immobilized with anti-human CD61 antibody. Anal Chim Acta. 2019;1091:160–8.
Acknowledgements
This research was partially supported by KAKENHI grants (21H02005, 21H05027, 23H04088) from the Japan Society for the Promotion of Science (JSPS), JSPS Bilateral Joint Research Projects (JPJSBP12022359, JPJSBP120203510), the Cooperative Research Program of “Network Joint Research Center for Materials and Devices (20234041),” and MEXT Promotion of Distinctive Joint Research Center Program (JPMXP 0621467946).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takagi, K., Bhowmik, S., Huang, KT. et al. Complex formation of pendant lysine residue-containing zwitterionic random copolymer with copper (II). Polym J 55, 1075–1083 (2023). https://doi.org/10.1038/s41428-023-00808-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41428-023-00808-2