Abstract
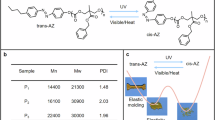
The photoinduced solid‒liquid phase transition is a fascinating phenomenon that can be utilized for a range of applications, including debondable adhesives, photolithography, and soft actuators; however, developing polymers with this function is not trivial. In this work, we report an azobenzene (Azo)-containing polymer capable of rapid room-temperature photoliquefaction upon UV irradiation and elucidate the design principles for photoliquefying polymers that harness the photothermal effect. We prepare a series of Azo polymers by coupling diacrylate Azo with dithiol-functionalized flexible spacers of different lengths, such as ethylene glycol (EG), hexa(ethylene glycol) (HEG), and poly(ethylene glycol) (PEG). EG-Azo, with the shortest spacer, has a high melting temperature (Tm) of 78 °C due to the strong interactions among the liquid-crystalline Azo molecules. Owing to the high Tm, EG-Azo does not exhibit a photoinduced solid‒liquid phase transition, although it has the greatest photothermal effect among the polymers (temperature rise to 50 °C). The incorporation of the longer spacers effectively decreases the Tm of the Azo polymers. For example, PEG-Azo possesses a reduced Tm of 40 °C, thereby enabling photoliquefaction at room temperature after only 1 min of UV irradiation. PEG-Azo can be reversibly returned to a solid-state within 5 min after the UV light is turned off.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Xu W-C, Sun S, Wu S. Photoinduced reversible solid‐to‐liquid transitions for photoswitchable materials. Angew Chem Int Ed 2019;58:9712–40.
Bisoyi HK, Li Q. Light-driven liquid crystalline materials: from photo-induced phase transitions and property modulations to applications. Chem Rev 2016;116:15089–166.
Zhou H, Kuenstler AS, Xu W, Hu M, Hayward RC. A semicrystalline poly (azobenzene) exhibiting room temperature light-induced melting, crystallization, and alignment. Macromolecules. 2022;55:10330–40.
Wu Z, Ji C, Zhao X, Han Y, Müllen K, Pan K, et al. Green-light-triggered phase transition of azobenzene derivatives toward reversible adhesives. J Am Chem Soc. 2019;141:7385–90.
Yue Y, Norikane Y, Azumi R, Koyama E. Light-induced mechanical response in crosslinked liquid-crystalline polymers with photoswitchable glass transition temperatures. Nat Commun 2018;9:3234.
Zhang P, Cai F, Wang W, Wang G, Yu H. Light-switchable adhesion of azobenzene-containing siloxane-based tough adhesive. ACS Appl Polym Mater 2021;3:2325–9.
Ito S, Akiyama H, Sekizawa R, Mori M, Yoshida M, Kihara H. Light-induced reworkable adhesives based on ABA-type triblock copolymers with azopolymer termini. ACS Appl Mater Interfaces. 2018;10:32649–58.
Zha RH, Vantomme G, Berrocal JA, Gosens R, de Waal B, Meskers S, et al. Photoswitchable nanomaterials based on hierarchically organized siloxane oligomers. Adv Funct Mater 2018;28:1703952.
Norikane Y, Uchida E, Tanaka S, Fujiwara K, Koyama E, Azumi R, et al. Photoinduced crystal-to-liquid phase transitions of azobenzene derivatives and their application in photolithography processes through a solid–liquid patterning. Org Lett 2014;16:5012–5.
Carroll GT, Lee KM, McConney ME, Hall HJ. Optical control of alignment and patterning in an azobenzene liquid crystal photoresist. J Mater Chem C. 2023;11:2177–85.
Zhou H, Xue C, Weis P, Suzuki Y, Huang S, Koynov K, et al. Photoswitching of glass transition temperatures of azobenzene-containing polymers induces reversible solid-to-liquid transitions. Nat Chem 2017;9:145–51.
Bandara HMD, Burdette SC. Photoisomerization in different classes of azobenzene. Chem Soc Rev 2012;41:1809–25.
Uchida E, Sakaki K, Nakamura Y, Azumi R, Hirai Y, Akiyama H, et al. Control of the orientation and photoinduced phase transitions of macrocyclic azobenzene. Chem Eur J 2013;19:17391–7.
Norikane Y, Hirai Y, Yoshida M. Photoinduced isothermal phase transitions of liquid-crystalline macrocyclic azobenzenes. Chem Commun 2011;47:1770–2.
de Haan LT, Schenning APHJ, Broer DJ. Programmed morphing of liquid crystal networks. Polymer 2014;55:5885–96.
Yu H. Recent advances in photoresponsive liquid-crystalline polymers containing azobenzene chromophores. J Mater Chem C. 2014;2:3047–54.
Yang B, Cai F, Huang S, Yu H. Athermal and soft multi-nanopatterning of azopolymers: phototunable mechanical properties. Angew Chem Int Ed 2020;59:4035–42.
Hartley GS. The cis-form of azobenzene. Nature. 1937;140:281–281.
Tazuke S, Kurihara S, Ikeda T. Amplified image recording in liquid crystal media by means of photochemically triggered phase transition. C. Chem Lett 2006;16:911–4.
Pang X, Lv J-A, Zhu C, Qin L, Yu Y. Photodeformable azobenzene‐containing liquid crystal polymers and soft actuators. Adv Mater 2019;31:1904224.
Lee C, Ndaya D, Bosire R, Kim NK, Kasi RM, Osuji CO. Fast photoswitchable order–disorder transitions in liquid-crystalline block co-oligomers. J Am Chem Soc 2022;144:390–9.
Yang Y, Huang S, Ma Y, Yi J, Jiang Y, Chang X, et al. Liquid and photoliquefiable azobenzene derivatives for solvent-free molecular solar thermal fuels. ACS Appl Mater Interfaces. 2022;14:35623–34.
Yang Q, Ge J, Qin M, Wang H, Yang X, Zhou X, et al. Controllable heat release of phase-change azobenzenes by optimizing molecular structures for low-temperature energy utilization. Sci China-Mater 2023;66:3609–20.
Feng W, Luo W, Feng Y. Photo-responsive carbon nanomaterials functionalized by azobenzene moieties: structures, properties and application. Nanoscale. 2012;4:6118–34.
Kuang Z-Y, Deng Y, Hu J, Tao L, Wang P, Chen J, et al. Responsive smart windows enabled by the azobenzene copolymer brush with photothermal effect. ACS Appl Mater Interfaces. 2019;11:37026–34.
Chen Y, Yu H, Quan M, Zhang L, Yang H, Lu Y. Photothermal effect of azopyridine compounds and their applications. RSC Adv. 2015;5:4675–80.
Da Cunha MP, van Thoor EAJ, Debije MG, Broer DJ, Schenning APHJ. Unravelling the photothermal and photomechanical contributions to actuation of azobenzene-doped liquid crystal polymers in air and water. J Mater Chem C. 2019;7:13502–9.
Lahikainen M, Zeng H, Priimagi A. Reconfigurable photoactuator through synergistic use of photochemical and photothermal effects. Nat Commun 2018;9:4148.
Guo Y, Xiao J, Sun Y, Song B, Zhang H, Dong B. Photoswitching of the melting point of a semicrystalline polymer by the azobenzene terminal group for a reversible solid-to-liquid transition. J Mater Chem A. 2021;9:9364–70.
Lee KM, White TJ. Photochemical mechanism and photothermal considerations in the mechanical response of monodomain, azobenzene-functionalized liquid crystal polymer networks. Macromolecules. 2012;45:7163–70.
Lamarre L, Sung CSP. Studies of physical aging and molecular motion by azochromophoric labels attached to the main chains of amorphous polymers. Macromolecules. 1983;16:1729–36.
Algers J, Sperr P, Egger W, Liszkay L, Kögel G, de Baerdemaeker J, et al. Free volume determination of azobenzene−PMMA copolymer by a pulsed low-energy positron lifetime beam with in-situ UV illumination. Macromolecules. 2004;37:8035–42.
Wang C, Weiss RG. Thermal cis → trans isomerization of covalently attached azobenzene groups in undrawn and drawn polyethylene films. Characterization and comparisons of occupied sites. Macromolecules. 2003;36:3833–40.
Dong L, Zhai F, Wang H, Peng C, Feng Y, Feng W. An azobenzene-based photothermal energy storage system for co-harvesting photon energy and low-grade ambient heat via a photoinduced crystal-to-liquid transition. Energy Mater. 2022;2:200025.
Urner LH, Thota BNS, Nachtigall O, Warnke S, von Helden G, Haag R, et al. Online monitoring the isomerization of an azobenzene-based dendritic bolaamphiphile using ion mobility-mass spectrometry. Chem Commun 2015;51:8801–4.
Tsutsumi O, Shiono T, Ikeda T, Galli G. Photochemical phase transition behavior of nematic liquid crystals with azobenzene moieties as both mesogens and photosensitive chromophores. J Phys Chem B. 1997;101:1332–7.
Asano T, Okada T, Thermal ZE. isomerization of azobenzenes. The pressure, solvent, and substituent effects. J Org Chem. 1984;49:4387–91.
Yang SY, Kim JG, Heo YD, Choe YS. A study of the isomerization reaction rates of azobenzene derivatives. J Korean Chem Soc. 1994;38:552–61.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIT) (RS-2023-00212143). This work was also supported by the H2KOREA funded by the Ministry of Education (2022Hydrogen fuel cell-003, Innovation Human Resources Development Project for Hydrogen Fuel Cells). This work was also supported by the Chung-Ang University Research Scholarship Grants in 2022. We would like to thank Prof. Dae Seok Kim and Hye Joo Lee at Pukyong National University for their help with the GPC measurements.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, Y., Kim, D., Lee, W. et al. Role of flexible spacers in achieving photoinduced phase transitions of azobenzene-based liquid-crystalline polymers at room temperature. Polym J 56, 1061–1067 (2024). https://doi.org/10.1038/s41428-024-00946-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41428-024-00946-1