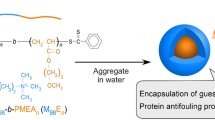
Abstract
This study prepared dual thermoresponsive diblock copolymers (E95Nn; n = 93 and 291) comprising poly(ethylene glycol) ethyl ether acrylate (PeDEGA; E) and poly(N-isopropylacrylamide) (PNIPAM; N) blocks with different lower critical solution temperatures (LCSTs). E95Nn was prepared via organotellurium-mediated living radical polymerization through a one-pot synthesis method. Energy-dispersive X-ray spectroscopy revealed that tellurium residue at the polymer chain end was removed during purification via dialysis. The LCST of the PeDEGA was lower than that of PNIPAM. At temperatures below the LCST of PeDEGA, E95Nn dissolved as a single polymer chain (the unimer state). When an aqueous solution of E95Nn was heated, polymer micelles with a PeDEGA core and PNIPAM shells formed above the LCST of the PeDEGA. In pure water, 7–10 polymer micelles formed intermicellar aggregates. The polymer micelles encapsulated hydrophobic guest molecules into the hydrophobic core formed from the PeDEGA chains. Large intermicellar aggregates formed above the LCST of PNIPAM owing to hydrophobic interactions between the PNIPAM shells. It is expected that E95Nn polymer micelles can be applied as drug carriers for thermoresponsive controlled drug release.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
Bordat A, Boissenot T, Nicolas J, Tsapis N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv Drug Deliv Rev. 2019;138:167–92.
Ward MA, Georgiou TK. Thermoresponsive polymers for biomedical applications. Polymers. 2011;3:1215–42.
Vancoillie G, Frank D, Hoogenboom R. Thermoresponsive poly(oligo ethylene glycol acrylates). Prog Polym Sci. 2014;39:1074–95.
Tanaka F, Koga T, Kojima H, Winnik FM. Temperature- and tension-induced coil-globule transition of poly(N-isopropylacrylamide) chains in water and mixed solvent of water/methanol. Macromolecules. 2009;42:1321–30.
Heskins M, Guillet JE. Solution properties of poly(N-isopropylacrylamide). J Macromol Sci Chem A. 1968;2:1441–55.
Giaouzi D, Pispas S. Synthesis and self-assembly of thermoresponsive poly(N -isopropylacrylamide)- b -poly(oligo ethylene glycol methyl ether acrylate) double hydrophilic block copolymers. Polym Chem. 2019;57:1467–77.
Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–92.
Maeda H. Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjug Chem. 2010;21:797–802.
Park J, Choi Y, Chang H, Um W, Ryu JH, Kwon IC. Alliance with EPR effect: Combined strategies to improve the EPR effect in the tumor microenvironment. Theranostics. 2019;9:8073–90.
Maeda H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J Control Release. 2012;164:138–44.
Goto A, Kwak Y, Fukuda T, Yamago S, Iida K, Nakajima M, et al. Mechanism-based invention of high-speed living radical polymerization using organotellurium compounds and azo-initiators. J Am Chem Soc. 2003;125:8720–1.
Chiefari J, Chong YK, Ercole F, Krstina J, Jeffery J, Le TPT, et al. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecules. 1998;31:5559–62.
Kwak Y, Goto A, Tsujii Y, Murata Y, Komatsu K, Fukuda T. A kinetic study on the rate retardation in radical polymerization of styrene with addition−fragmentation chain transfer. Macromolecules. 2002;35:3026–9.
Calitz FM, McLeary JB, McKenzie JM, Tonge MP, Klumperman B, Sanderson RD. Evidence for termination of intermediate radical species in RAFT-mediated polymerization. Macromolecules. 2003;36:9687–90.
Yamago S. Development of organotellurium-mediated and organostibine-mediated living radical polymerization reactions. J Polym Sci A. 2006;44:1–12.
Yamago S. Precision polymer synthesis by degenerative transfer controlled/living radical polymerization using organotellurium, organostibine, and organobismuthine chain-transfer agents. Chem Rev. 2009;109:5051–68.
Kayahara E, Yamago S, Kwak Y, Goto A, Fukuda T. Optimization of organotellurium transfer agents for highly controlled living radical polymerization. Macromolecules. 2008;41:527–9.
Kleks G, Holland DC, Porter J, Carroll AR. Natural products dereplication by diffusion ordered NMR spectroscopy (DOSY). Chem Sci. 2021;12:10930–43.
Kitano K, Ishihara K, Yusa SI. Preparation of a thermo-responsive drug carrier consisting of a biocompatible triblock copolymer and fullerene. J Mater Chem B. 2022;10:2551–60.
Matsumura K, Hyon SH. Polyampholytes as low toxic efficient cryoprotective agents with antifreeze protein properties. Biomaterials. 2009;30:4842–9.
Yamago S, Yamada T, Togai M, Ukai Y, Kayahara E, Pan N. Synthesis of structurally well-defined telechelic polymers by organostibine-mediated living radical polymerization: In situ generation of functionalized chain-transfer agents and selective omega-end-group transformations. Chem Eur J. 2009;15:1018–29.
Pelton R. Poly(N-isopropylacrylamide) (PNIPAM) is never hydrophobic. J Colloid Interface Sci. 2010;348:673–4.
Jean B, Lee L-T, Cabane B. Interactions of sodium dodecyl sulfate with acrylamide – N -isopropylacrylamide) statistical copolymer. Colloid Polym Sci. 2000;278:764–70.
Tanaka F, Okada Y. Theoretical study on the phase diagrams of associating polymers. Netsu Sokutei. 2005;32:178–85.
Pedersen JS, Gerstenberg MC. Scattering form factor of block copolymer micelles. Macromolecules. 1996;29:1363–65.
Pedersen JS. Structure factors effects in small-angle scattering from block copolymer micelles and star polymers. J Chem Phys. 2001;114:2839–46.
Kajiwara K, Burchard W, Gordon M. Angular distribution of Rayleigh scattering from randomly branched polycondensates. Br Polym J. 1970;2:110–5.
Koberstein JT, Morra B, Stein RS. The determination of diffuse-boundary thicknesses of polymers by small-angle X-ray scattering. J Appl Crystallogr. 1980;13:34–45.
Burchard W, Schmidt M, Stockmayer WH. Information on polydispersity and branching from combined quasi-elastic and intergrated scattering. Macromolecules. 1980;13:1265–72.
Konishi T, Yoshizaki T, Yamakawa H. On the “universal constants” ρ and Φ. of flexible polymers. Macromolecules. 1991;24:5614–22.
Zhang Y, Furyk S, Bergbreiter DE, Cremer PS. Specific ion effects on the water solubility of macromolecules: PNIPAM and the Hofmeister series. J Am Chem Soc. 2005;127:14505–10.
Overath P, Träuble H. Phase transitions in cells, membranes, and lipids of Escherichia coli. Detection by fluorescent probes, light scattering, and dilatometry. Biochemistry. 1973;12:2625–34.
Kumar N, Oqmhula K, Hongo K, Takagi K, Yusa SI, Rajan R, et al. Mechanistic insights and importance of hydrophobicity in cationic polymers for cancer therapy. J Mater Chem B. 2023;11:1456–68.
Acknowledgements
This research was partially supported by KAKENHI grants (21H02005, 23H04088, 21H05027) from the Japan Society for the Promotion of Science (JSPS), JSPS Bilateral Joint Research Projects (JPJSBP12022359, JPJSBP120203510), the Cooperative Research Program of the Network Joint Research Center for Materials and Devices (20,234,041), and the MEXT Promotion of Distinctive Joint Research Center Program (JPMXP 0621467946). The SAXS experiments were carried out at Spring-8 under the approval of JASRI. We would like to thank KJ Chemical for the gift of NIPAM. We would also like to thank Otsuka Chemical for the gift of BTEE.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hayashi, M., Takahashi, R., Vu, T.N. et al. Thermoresponsive behavior of dual hydrophilic diblock copolymers prepared via organotellurium-mediated living radical polymerization. Polym J 56, 1129–1141 (2024). https://doi.org/10.1038/s41428-024-00952-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41428-024-00952-3