Abstract
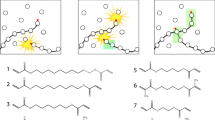
The thermal curing reactions of p-tert-butylcalix[n]arenes (Cn) (n = 4, 6, and 8) with 1,3-phenylenebis(2-oxazoline) (PBO) were performed. The obtained thermosets were characterized to determine the relationships between the ring size of the calixarenes and the properties of their network polymers. The samples were cured by heating at 160 °C and 180 °C for 1 h each and then at 200 °C, 230 °C, and 250 °C for 2 h each without a solvent and catalyst. For comparison, a corresponding linear four-nucleus novolac (L4) was cured with PBO under the same conditions. Dynamic mechanical analyses of the thermosets revealed that the glass transition temperature (Tg) increased in the following order: L4/PBO < C4/PBO < C8/PBO < C6/PBO. Model reactions with a monofunctional oxazoline compound indicated that the final crosslinking degree of the network polymers increased with increasing ring size. Conversely, the cyclic structures became increasingly rigid as the ring size decreased. Because of its moderate reactivity and rigidity, the network polymer derived from C6 exhibited the highest Tg.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Ogata M, Kinjo N, Eguchi S, Hozoji H, Kawata T, Sashima H. Effects of curing accelerators on physical properties of epoxy molding compound (EMC). J Appl Polym Sci. 1992;44:1795–805.
Ogata M, Kinjo N, Kawata T. Effects of crosslinking on physical properties of phenol–formaldehyde novolac cured epoxy resins. J Appl Polym Sci. 1993;48:583–601.
Matsumoto A, Hasegawa K, Fukuda A. Phenol novolac/poly(4-hydroxyphenylmaleimide) blend hardeners for DGEBA-type epoxy resin. Polym Int. 1992;28:173–7.
Matsumoto A, Hasegawa K, Fukuda A, Pae JS. Properties of epoxy resin cured by phenol novolac/4-hydroxyphenylmaleimide polymer blend hardeners. Polym Int. 1993;31:275–82.
Culbertson BM, Tiba O, Deviney ML. Thermosetting bisoxazoline-phenolic resin matrix materials for aerospace industry applications. Polym Mater Sci Eng. 1988;59:830–4.
Culbertson BM, Tong Y, Schricker SR. Synthesis and ring-opening reactions of tetraphenyl-substituted bisoxazoline monomers for preparing new thermosets and composites. Polym Adv Technol. 2000;11:9–14.
Tong Y, Gao F, Huang Y, Schricker SR, Culbertson BM. Synthesis of ether-linked bisoxazolines and their use in step-growth polymerization reactions with phenolics. Polym Adv Technol. 2002;13:311–9.
Kimura H, Matsumoto A, Hasegawa K, Fukuda A. New thermosetting resin from bisphenol A-based benzoxazine and bisoxazoline. J Appl Polym Sci. 1999;72:1551–8.
Hasegawa K, Fukuda A, Tonogai S. Structure and viscoelastic properties of epoxy resins prepared from four-nuclei novolacs. J Appl Polym Sci. 1989;37:3423–35.
Hasegawa K, Fukuda A, Tonogai S, Uede K. Structure and viscoelastic properties of epoxy resins prepared from o-cresol novolacs. J Appl Polym Sci. 1989;38:1581–90.
Iijima T, Kabaya H, Tomoi M. Synthesis and properties of a new o-cresol novolak epoxy resin containing oxyethylene units. Angew Makromol Chem. 1990;181:199–205.
Ohtsuka K, Hasegawa K, Fukuda A, Uede K. Curing behavior of epoxy resin having hydroxymethyl group and different molecular weight distribution. J Appl Polym Sci. 1992;44:1543–6.
Pan G, Du Z, Zhang C, Li C, Yang X, Li H. Effect of structure of bridging group on curing and properties of bisphenol-A based novolac epoxy resins. Polym J. 2007;39:478–87.
Gutsche CD, Iqbal M. p-tert-Butylcalix[4]arene. Org Synth. 1990;68:234–6.
Gutsche CD, Dhawan B, Leonis M, Stewart D. p-tert-Butylcalix[6]arene. Org Synth. 1990;68:238–40.
Munch JH, Gutsche CD. p-tert-Butylcalix[8]arene. Org Synth. 1990;68:243–5.
Nishikubo T, Kameyama A, Kudo H. Novel high performance materials. Calixarene derivatives containing protective groups and polymerizable groups for photolithography, and calixarene derivatives containing active ester groups for thermal curing of epoxy resins. Polym J. 2003;35:213–29.
Xu S, Kudo H, Nishikubo T, Nakamura S, Numata S. Thermal cured epoxy resins using certain calixarenes and their esterified derivatives as curing agents. J Polym Sci Part A Polym Chem. 2010;48:1931–42.
Sugioka T, Hay AS. Synthesis of fully lower-rim, carbonate-bridged calix[8]arenes and their curing behavior. J Polym Sci Part A Polym Chem. 2001;39:1149–55.
Jeon YM, Kim SH, Gong MS. Preparation and properties of calix[4]arene-based epoxy resins. Macromol Res. 2005;13:553–6.
Shetty D, Jahovic I, Raya J, Ravaux F, Jouiad M, Olsen JC, et al. An ultra-absorbent alkyne-rich porous covalent polycalix[4]arene for water purification. J Mater Chem A. 2017;5:62–66.
Ning Y, Chen Y, Wang M, Zhou K, Su T, Wang Z. Calixarene-based cyanate ester resin for high-temperature material. High Perform Polym. 2019;31:359–66.
Yonekawa M, Kimura H, Ohtsuka K. Thermal, mechanical, and dielectric characterization of p-tert-butylcalix[8]arene glycidyl ether/benzoxazine copolymer. Chem Lett. 2020;49:601–4.
Dhawan B, Gutsche CD. Calixarenes. 10. oxacalixarenes. J Org Chem. 1983;48:1536–9.
Ingenfeld B, Straub S, Frömbgen C, Lützen A. Synthesis of monofunctionalized calix[5]arenes. Synthesis. 2018;50:676–84.
Sano Y. Polymerization of bis(2-oxazoline) compounds with dicarboxylic acids. J Polym Sci Part A Polym Chem 1989;27:2749–60.
Acknowledgements
This work was supported by JSPS KAKENHI grant number 18K14293.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yonekawa, M., Kimura, H., Ohtsuka, K. et al. Effect of the cyclic structures of p-tert-butylcalix[n]arenes on a bisoxazoline curing system. Polym J 57, 87–94 (2025). https://doi.org/10.1038/s41428-024-00964-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41428-024-00964-z