Abstract
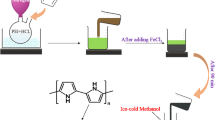
Surfactant-free coupling polymerization of pyrrole (Py) and its derivatives, namely, N-methylpyrrole (MPy) and N-ethylpyrrole (EPy), was conducted using solid Fe(NO3)3 in the presence of an aqueous medium, resulting in aqueous dispersions of polymer particles. Dynamic light scattering studies revealed the production of colloidally stable polymer nanoparticles with diameters of 153–206 nm, 262–294 nm and 273–278 nm in aqueous media for the Py, MPy and EPy systems, respectively. The particle sizes of poly(N-methylpyrrole) (PMPy) and poly(N-ethylpyrrole) (PEPy) were larger than those of polypyrrole (PPy), which could be due to the greater hydrophobicity of MPy and EPy than Py. The particles could achieve colloidal stability through an electrostatic stabilization mechanism, as the polymerization process introduces cationic charges to the polymers via doping. Larger amounts of hydroxy and carbonyl groups were introduced into PMPy and PEPy because of the easier overoxidation of MPy and EPy due to their lower redox potentials than that of Py. Furthermore, the resulting particles could adsorb on oil‒water interfaces and work as effective Pickering-type emulsifiers. Suspension polymerization of vinyl monomer-in-water Pickering emulsions stabilized with PPy and PMPy nanoparticles resulted in the production of nanoparticle-coated polymer microparticles with diameters of 25 μm and 154 μm, respectively.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Angeli A. Sopra il nero del pirrolo. Nota preliminare. Atti Accad Naz Lincei Cl Sci Fis Mat Re 1915;24:3–6.
Rasmussen SC. Conjugated and Conducting Organic Polymers: The First 150 Years. Chempluschem. 2020;85:1412–29.
Skotheim TA, Elsenbaumer RL, Gardiner JR. Handbook of Conducting Polymers, 2nd ed. Boulder: NetLibrary, Incorporated; 1998, pp. 1–1120.
Guimard NK, Gomez N, Schmidt CE. Conducting Polymers in Biomedical Engineering. Prog Polym Sci. 2007;32:876–921.
Balint R, Cassidy NJ, Cartmell SH. Conductive polymers: towards a smart biomaterial for tissue engineering. Acta Biomater. 2014;10:2341–53.
Ateh DD, Navsaria HA, Vadgama P. Polypyrrole-based conducting polymers and interactions with biological tissues. J R Soc Interface. 2006;3:741–52.
Fielding LA, Hillier JK, Burchell MJ, Armes SP. Space science applications for conducting polymer particles: synthetic mimics for cosmic dust and micrometeorites. Chem Commun. 2015;51:16886–99.
Ramanavičius A, Ramanavičienė A, Malinauskas A. Electrochemical sensors based on conducting polymer—polypyrrole. Electrochim Acta. 2006;51:6025–37.
Setka M, Drbohlavova J, Hubalek J. Nanostructured Polypyrrole-Based Ammonia and Volatile Organic Compound Sensors. Sensors. 2017;17:562.
Melling D, Martinez JG, Jager EWH. Conjugated Polymer Actuators and Devices: Progress and Opportunities. Adv Mater. 2019;31:1808210.
Jakobs RCM, Janssen LJJ, Barendrecht E. Hydroquinone oxidation and p-benzoquinone reduction at polypyrrole and poly-N-methylpyrrole electrodes. Electrochim Acta. 1985;30:1313–21.
Nezakati T, Seifalian A, Tan A, Seifalian AM. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem Rev. 2018;118:6766–843.
Li F, Winnik MA, Matvienko A, Mandelis A. Polypyrrole Nanoparticles as a Thermal Transducer of NIR Radiation in Hot-Melt Adhesives. J Mater Chem. 2007;17:4309–15.
Chen M, Fang X, Tang S, Zheng N. Polypyrrole nanoparticles for high-performance in vivo near-infrared photothermal cancer therapy. Chem Commun. 2012;48:8934–6.
Takeuchi M, Kawashima H, Imai H, Fujii S, Oaki Y. Quantitative detection of near-infrared (NIR) light using organic layered composites. J Mater Chem C. 2019;7:4089–95.
Liu Y, Xiong H, Huang H, Li L, Huang Y, Yu X. Fabrication of poly(N-methylpyrrole) nanotubes for detection of dopamine. Polym Bull. 2018;75:2357–68.
Prezyna LA, Qiu YJ, Reynolds JR, Wnek GE. Interaction of cationic polypeptides with electroactive polypyrrole/poly(styrenesulfonate) and poly(N-methylpyrrole)/poly(styrenesulfonate) films. Macromolecules. 1991;24:5283–7.
Elibal F, Gumustekin S, Ozkazanc H, Ozkazanc E. Poly(N-methylpyrrole) with high antibacterial activity synthesized via interfacial polymerization method. J Mol Struct. 2021;1242:130712.
Duran B, Bereket G. Cyclic Voltammetric Synthesis of Poly(N-methyl pyrrole) on Copper and Effects of Polymerization Parameters on Corrosion Performance. Ind Eng Chem Res. 2012;51:5246–55.
Kou C-T, Liou T-R. Characterization of metal—oxide—semiconductor field-effect transistor (MOSFET) for polypyrrole and poly (N-alkylpyrrole)s prepared by electrochemical synthesis. Synth Met. 1996;82:167–73.
Hao L, Dong C, Yu D. Polypyrrole Derivatives: Preparation, Properties and Application. Polymers. 2024;16:2233.
Bjorklund RB, Liedberg B. Electrically conducting composites of colloidal polypyrrole and methylcellulose. J Chem Soc Chem Commun. 1986;1293–5.
Markham G, Obey TM, Vincent B. The preparation and properties of dispersions of electrically-conducting polypyrrole particles. Colloids Surf. 1990;51:239–53.
Armes SP, Aldissi M. Preparation and characterization of colloidal dispersions of polypyrrole using poly(2-vinyl pyridine)-based steric stabilizers. Polymer. 1990;31:569–74.
Mrkic J, Saunders BR. Microgel Particles as a Matrix for Polymerization: A Study of Poly(N-isopropylacrylamide)-Poly(N-methylpyrrole) Dispersions. J Colloid Interface Sci. 2000;222:75–82.
Alizadeh N, Akbarinejad A. Soluble fluorescent polymeric nanoparticles based on pyrrole derivatives: synthesis, characterization and their structure dependent sensing properties. J Mater Chem C. 2015;3:9910–20.
Armes SP. Conducting polymer colloids. Curr Opin Colloid Interface Sci. 1996;1:214–20.
Fujii S. Synthesis of Conductive Polymer-Based Composite Particles. Chem Lett. 2023;52:631–9.
Kawashima H, Mayama H, Nakamura Y, Fujii S. Hydrophobic Polypyrroles Synthesized by Aqueous Chemical Oxidative Polymerization and Their Use as Light-Responsive Liquid Marble Stabilizers. Polym Chem. 2017;8:2609–18.
Asaumi Y, Rey M, Oyama K, Vogel N, Hirai T, Nakamura Y, et al. Effect of Stabilizing Particle Size on the Structure and Properties of Liquid Marbles. Langmuir. 2020;36:13274–84.
Asaumi Y, Rey M, Vogel N, Nakamura Y, Fujii S. Particle Monolayer-Stabilized Light-Sensitive Liquid Marbles from Polypyrrole-Coated Microparticles. Langmuir. 2020;36:2695–706.
Du L, Gao P, Meng Y, Liu Y, Le S, Yu C. Highly Efficient Removal of Cr(VI) from Aqueous Solutions by Polypyrrole/Monodisperse Latex Spheres. ACS Omega. 2020;5:6651–60.
Muhammad Ekramul Mahmud HN, Huq AKO, Yahya RB. The removal of heavy metal ions from wastewater/aqueous solution using polypyrrole-based adsorbents: a review. RSC Adv. 2016;6:14778–91.
Au KM, Lu Z, Matcher SJ, Armes SP. Polypyrrole nanoparticles: a potential optical coherence tomography contrast agent for cancer imaging. Adv Mater. 2011;23:5792–5.
Pope MR, Armes SP, Tarcha PJ. Specific Activity of Polypyrrole Nanoparticulate Immunoreagents: Comparison of Surface Chemistry and Immobilization Options. Bioconjugate Chem. 1996;7:436–44.
Kim SW, Cho HG, Park CR. Fabrication of unagglomerated polypyrrole nanospheres with controlled sizes from a surfactant-free emulsion system. Langmuir. 2009;25:9030–6.
Vetter CA, Suryawanshi A, Lamb JR, Law B, Gelling VJ. Novel synthesis of stable polypyrrole nanospheres using ozone. Langmuir. 2011;27:13719–28.
Cui Z, Coletta C, Dazzi A, Lefrancois P, Gervais M, Neron S, et al. Radiolytic method as a novel approach for the synthesis of nanostructured conducting polypyrrole. Langmuir. 2014;30:14086–94.
Liao Y, Li X-G, Kaner RB. Facile Synthesis of Water-Dispersible Conducting Polymer Nanospheres. ACS Nano. 2010;4:5193–202.
Liao Y, Wang X, Qian W, Li Y, Li X, Yu DG. Bulk synthesis, optimization, and characterization of highly dispersible polypyrrole nanoparticles toward protein separation using nanocomposite membranes. J Colloid Interface Sci. 2012;386:148–57.
Li X-G, Li A, Huang M-R, Liao Y, Lu Y-G. Efficient and Scalable Synthesis of Pure Polypyrrole Nanoparticles Applicable for Advanced Nanocomposites and Carbon Nanoparticles. J Phys Chem C. 2010;114:19244–55.
Atsuta Y, Takeuchi K, Shioda N, Hamada W, Hirai T, Nakamura Y, et al. Colloidally Stable Polypyrrole Nanoparticles Synthesized by Surfactant-Free Coupling Polymerization. Langmuir. 2023;39:14984–95.
Muramatsu R, Oaki Y, Kuwabara K, Hayashi K, Imai H. Solvent-free synthesis, coating and morphogenesis of conductive polymer materials through spontaneous generation of activated monomers. Chem Commun. 2014;50:11840–3.
Kuwabara K, Masaki H, Imai H, Oaki Y. Substrate coating by conductive polymers through spontaneous oxidation and polymerization. Nanoscale. 2017;9:7895–7900.
K N, Rout CS. Conducting polymers: a comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021;11:5659–97.
Lovell PA, El-Aasser MS. Emulsion Polymerization and Emulsion Polymers. Wiley: New York; 1997, pp. 1–826.
Hansen CM. The three dimensional solubility parameter - key to paint component affinities: I. Solvents, plasticizers, polymers, and resins. J Paint Technol. 1967;39:104–17.
Pei Q, Qian R. Protonation and deprotonation of polypyrrole chain in aqueous solutions. Synth Met. 1991;45:35–48.
Zhang X, Bai. Surface Electric Properties of Polypyrrole in Aqueous Solutions. Langmuir. 2003;19:10703–9.
Greenwood R. Review of the measurement of zeta potentials in concentrated aqueous suspensions using electroacoustics. Adv Colloid Interface Sci. 2003;106:55–81.
Feeney PJ, Napper DH, Gilbert RG. Surfactant-free emulsion polymerizations: predictions of the coagulative nucleation theory. Macromolecules. 1987;20:2922–30.
Lovell PA, Schork FJ. Fundamentals of Emulsion Polymerization. Biomacromolecules. 2020;21:4396–441.
Ayad MM. In-situ polypyrrole film formation using ferric nitrate as oxidizing agent. J Mater Sci Lett. 2003;22:1577–9.
Fujii S, Aichi A, Akamatsu K, Nawafune H, Nakamura Y. One-step synthesis of polypyrrole-coated silver nanocomposite particles and their application as a coloured particulate emulsifier. J Mater Chem. 2007;17:3777–9.
Omastová M, Trchová M, Kovářová J, Stejskal J. Synthesis and structural study of polypyrroles prepared in the presence of surfactants. Synth Met. 2003;138:447–55.
Ćirić-Marjanović G, Mentus S, Pašti I, Gavrilov N, Krstić J, Travas-Sejdic J, et al. Synthesis, Characterization, and Electrochemistry of Nanotubular Polypyrrole and Polypyrrole-Derived Carbon Nanotubes. J Phys Chem C. 2014;118:14770–84.
Tabačiarová J, Mičušík M, Fedorko P, Omastová M. Study of Polypyrrole Aging by XPS, FTIR and Conductivity Measurements. Polym Degrad Stab. 2015;120:392–401.
Seike M, Uda M, Suzuki T, Minami H, Higashimoto S, Hirai T, et al. Synthesis of Polypyrrole and Its Derivatives as a Liquid Marble Stabilizer via a Solvent-Free Chemical Oxidative Polymerization Protocol. ACS Omega. 2022;7:13010–21.
Lei J, Cai Z, Martin CR. Effect of Reagent Concentrations Used to Synthesize Polypyrrole on the Chemical Characteristics and Optical and Electronic Properties of the Resulting Polymer. Synth Met. 1992;46:53–69.
Mazumder A, Sebastian E, Hariharan M. Solvent dielectric delimited nitro-nitrito photorearrangement in a perylenediimide derivative. Chem Sci. 2022;13:8860–70.
Liang W, Lei J, Martin CR. Effect of Synthesis Temperature on the Structure, Doping Level and Charge-Transport Properties of Polypyrrole. Synth Met. 1992;52:227–39.
Malitesta C, Losito I, Sabbatini L, Zambonin PG. New findings on polypyrrole chemical structure by XPS coupled to chemical derivatization labelling. J Electron Spectrosc Relat Phenom. 1995;76:629–34.
Ramsden W, Gotch F. Separation of Solids in the Surface-Layers of Solutions and ‘Suspensions’ (Observations on Surface-Membranes, Bubbles, Emulsions, and Mechanical Coagulation).-Preliminary Account. Proc R Soc Lond. 1904;72:156–64.
Pickering SU. CXCVI.—Emulsions. J Chem Soc Trans. 1907;91:2001–21.
Binks BP, Horozov TS. Colloidal Particles at Liquid Interfaces. Cambridge, New York; Cambridge University Press: 2006, pp. 1–503.
Jiang H, Sheng Y, Ngai T. Pickering Emulsions: Versatility of Colloidal Particles and Recent Applications. Curr Opin Colloid Interface Sci. 2020;49:1–15.
Fameau A-L, Fujii S. Stimuli-Responsive Liquid Foams: From Design to Applications. Curr Opin Colloid Interface Sci. 2020;50:101380.
Subramaniam AB, Abkarian M, Mahadevan L, Stone HA. Colloid Science: Non-Spherical Bubbles. Nature. 2005;438:930.
Subramaniam AB, Abkarian M, Stone HA. Controlled assembly of jammed colloidal shells on fluid droplets. Nat Mater. 2005;4:553–6.
Fujii S, Nakamura Y. Stimuli-Responsive Bubbles and Foams Stabilized with Solid Particles. Langmuir. 2017;33:7365–79.
Fujii S. Foams/bubbles stabilized with polymer particles. Curr Opin Colloid Interface Sci. 2024;72:101808.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
Acknowledgements
The authors are grateful to Ms. Nano Shioda and Ms. Wakana Hamada (Keio Univ.) for conducting SEM and TEM studies.
Funding
This work was supported by Grants-in-Aid for Scientific Research (B) (JSPS KAKENHI Grant Numbers JP20H02803 and JP24K01562), a JSPS-DAAD Bilateral Joint Research Project (Grant Numbers JPJSBP120203509 and JPJSBP120223510), Challenging Research (Pioneering) (JSPS KAKENHI Grant Number JP22K19071), Scientific Research on Innovative Areas “New Polymeric Materials Based on Element-Blocks (JSPS KAKENHI Grant Number JP15H00767)” and “Aquatic Functional Materials: Creation of New Materials Science for Environment-Friendly and Active Functions (JSPS KAKENHI Grant Number JP22H04559)”, and a Private University Research Branding Project (Type A: Osaka Industrial Technology Platform).
Author information
Authors and Affiliations
Contributions
Yuya Atsuta: Methodology, Investigation. Kazusa Takeuchi: Methodology, Investigation. Tomoki Sakuma: Methodology. Koji Mitamura: Methodology. Seiji Watase: Methodology. Yuan Song: Methodology. Tomoyasu Hirai: Methodology, Investigation. Yoshinobu Nakamura: Methodology, Investigation. Yuya Oaki: Conceptualization, Methodology, Investigation, Writing - Review & Editing, Supervision, Project administration, Funding acquisition. Syuji Fujii: Conceptualization, Methodology, Investigation, Writing - Original Draft, Writing - Review & Editing, Supervision, Project administration, Funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Atsuta, Y., Takeuchi, K., Sakuma, T. et al. Synthesis of polypyrrole and its derivative nanoparticles via a surfactant-free coupling polymerization protocol. Polym J 57, 723–735 (2025). https://doi.org/10.1038/s41428-025-01026-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41428-025-01026-8
This article is cited by
-
Polypyrrole microtubes synthesized by vapor-phase coupling polymerization
Emergent Materials (2025)
-
Amendment in electrical and thermal properties of bismuth telluride with dispersion into conducting polymers nanocomposites
Applied Physics A (2025)