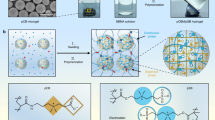
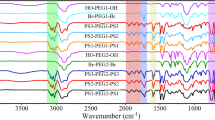
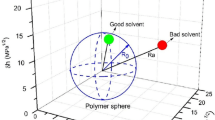
Abstract
An artificial kidney is a medical device used in dialysis treatment for patients with renal failure. Owing to its prolonged contact with blood, artificial kidneys must have excellent antithrombogenic properties. An artificial kidney contains approximately 10,000 porous hollow fiber membranes, which remove water and uremic toxins from the blood through the principles of dialysis and filtration. It is well known that hollow fiber membranes made from polysulfone-based polymers (PSf) and polyvinylpyrrolidone (PVP) have high removal performance, accounting for 90% of the global market share. PVP, a hydrophilic polymer, contributes to the expression of antithrombogenic properties. However, complications due to insufficient antithrombogenic properties remain a challenge, and there has been strong demand for further improvements in antithrombogenic properties. We succeeded in commercializing PSf membrane artificial kidneys containing antithrombogenic polymers other than PVP for the first time by focusing on the mobility of adsorbed water. Furthermore, advanced analysis made it possible to design antithrombogenic polymers driven by computational science. This review discusses the design of antithrombogenic polymers based on the mobility of water and the commercialization of antithrombogenic artificial kidneys.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
Amino M. Incidence of Thrombocytopenia Caused by the Use of Polysulfone Membranes. J Nagano Soc Dial Ther. 2008;31:p41.
Statistics of the Japanese Society for Dialysis Therapy. 1989.
Karkar A, Ronco C. Prescription of CRRT: a pathway to optimize therapy, Annuals of Intensive Care (Ann. Int. Care). 2020;10:32
Ishihara K, Nomura H, Mihara T, Kurita K, Iwasaki Y, Nakabayashi N. Why Do Phospholipid Polymers Reduce Protein Adsorption? J Biomed Mater Res. 1998;39:p323.
Tanaka M, Mochizuki A, Ishii N, Motomura T, Hatakeyama T. Study of Blood Compatibility with Poly(2-methoxyethyl acrylate). Relationship between Water Structure and Platelet Compatibility in Poly(2-methoxyethylacrylate-co-2-hydroxyethylmethacrylate). Biomacromol. 2002;3:p36.
Lu DR, Lee SJ, Park K. Interaction of Synthetic Polymer Surfaces with Proteins and Cells. J Biomater Sci Polym Ed. 1992;3:p127.
Nakada M, Yamada T, Ikeda K, Otomo T. Static and Dynamic Structure Analysis of Intermediate Water on Polyvinyl Pyrrolidone Using Neutron Scattering. JPS Conf Proc. 2021;33:011080.
Koga Y, Fujieda H, Meguro H, Ueno Y, Aoki T, Miwa K, et al. Biocompatibility of Polysulfone Hemodialysis Membranes and Its Mechanisms: Involvement of Fibrinogen and Its Integrin Receptors in Activation of Platelets and Neutrophils. Artif Organs. 2018;42:E246.
Koga Y, Meguro H, Fujieda H, Ueno Y, Miwa K, Kainoh M. A New Hydrophilic Polysulfone Hemodialysis Membrane Can Prevent Platelet-Neutrophil Interactions and Successive Neutrophil Activation. Int J Artif Organs. 2019;42:p175.
Yamaka T, Ichikawa K, Saito M, Watanabe K, Nakai A, Higuchi N, et al. Biocompatibility of the new anticoagulant dialyzer TORAYLIGHT® NV. Science Postprint, 2014;1:e00020.
Hidaka S, Kobayashi S, Maesato K, Mochida Y, Ishioka K, Oka M, et al. Hydrophilic Polymer-Coated Polysulfone Membrane Improves Endothelial Function of Hemodialysis Patients: A Pilot Study. J Clin Nephrol Res. 2015;2:1020.
Ronco C, Brendolan A, Nalesso F, Zanella M, De Cal M, Corradi V. Prospective, Randomized, Multicenter, Controlled Trial (TRIATHRON 1) on a New Antithrombogenic Hydrophilic Dialysis Membrane. Int J Artif Organs. 2017;40:p234.
Kodama H, Tsuji A, Fujinoki A, Ooshima K, Ishizeki K, Inoue T. Biocompatibility and Small Protein Permeability of Hydrophilic-Coated Membrane Dialyzer (NV) in Hemodialysis Patients: a Pilot Study. Renal Replace Ther. 2017;3:40.
Kakuta T, Komaba H, Takagi N, Takahashi Y, Suzuki H, Hyodo T, et al. A Prospective Multicenter Randomized Controlled Study on Interleukin-6 Removal and Induction by a New Hemodialyzer with Improved Biocompatibility in Hemodialysis Patients: A Pilot Study. Ther Apher Dial. 2016;20:p569.
Tsuchida K, Hashimoto H, Kawahara K, Hayashi I, Fukata Y, Kashiwagi M, et al. Effects of Hydrophilic Polymer-Coated Polysulfone Membrane Dialyzers on Intradialytic Hypotension in Diabetic Hemodialysis Patients (ATHRITE BP Study): a Pilot Study. Renal Replace Ther. 2017;3:58.
Kakuta T, Ishida M, Komaba H, Suzuki H, Fukagawa M. A Retrospective Study on Erythropoiesis Stimulating Agent Dose Reducing Potential of an Anti-Platelet Activation Membrane Dialyzer in Hemodialysis Patients. Ther Apher Dial. 2019;23:p133.
Acknowledgements
The authors would like to express their sincere gratitude to all those who collaborated and supported this research and its commercialization, as well as all medical professionals and patients using the resulting antithrombogenic artificial kidneys.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ueno, Y., Fujita, M., Sugaya, H. et al. Design of antithrombogenic polymers based on their interactions with water and commercialization of artificial kidneys. Polym J 58, 117–125 (2026). https://doi.org/10.1038/s41428-025-01045-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41428-025-01045-5