Abstract
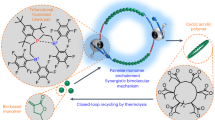
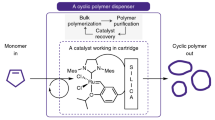
Macromolecules with cyclic topologies have attracted significant attention because of the absence of polymer chain ends that clearly distinguishes them from linear or branched polymers. However, the synthesis of macrocyclic polymers, particularly those possessing multiple cyclic units, remains challenging. Recently, our research group established a highly efficient method for the synthesis of multicyclic polymers without the use of cyclic precursors. This review provides a comprehensive overview of our recent studies and relevant research on the precise synthesis of multicyclic polymers, including cage-shaped polymers, spiro-multicyclic polymers, and graft polymers with macromolecular cyclic or cage side chains, via intramolecular ring-opening metathesis oligomerization or the cyclopolymerization of norbornenyl-functionalized macromonomers, mediated by third-generation Grubbs catalysts. In addition, their fundamental properties and potential applications are briefly discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



















Similar content being viewed by others
References
Clarson SJ, Semlyen JA. Cyclic polysiloxanes: 1. Preparation and characterization of poly(phenylmethylsiloxane). Polymer. 1986;27:1633–6.
Santangelo PG, Roland CM, Chang T, Cho D, Roovers J. Dynamics near the glass temperature of low molecular weight cyclic polystyrene. Macromolecules. 2001;34:9002–5.
Zimm BH, Stockmayer WH. The dimensions of chain molecules containing branches and rings. J Chem Phys. 1949;17:1301–14.
Morgese G, Trachsel L, Romio M, Divandari M, Ramakrishna SN, Benetti EM. Topological polymer chemistry enters surface science: linear versus cyclic polymer brushes. Angew Chem. 2016;128:15812–7.
Honda S, Yamamoto T, Tezuka Y. Topology-directed control on thermal stability: micelles formed from linear and cyclized amphiphilic block copolymers. J Am Chem Soc. 2010;132:10251–3.
Gavrilov M, Amir F, Kulis J, Hossain MD, Jia Z, Monteiro MJ. Densely packed multicyclic polymers. ACS Macro Lett. 2017;6:1036–41.
Pipertzis A, Hossain MD, Monteiro MJ, Floudas G. Segmental dynamics in multicyclic polystyrenes. Macromolecules. 2018;51:1488–97.
Ree BJ, Satoh Y, Isono T, Satoh T. Correlations of nanoscale film morphologies and topological confinement of three-Armed cage block copolymers. Polym Chem. 2021;12:3451–60.
Ree BJ, Satoh Y, Isono T, Satoh T. Influence of topological confinement on nanoscale film morphologies of tricyclic block copolymers. Macromolecules. 2021;54:4120–7.
Kyoda K, Yamamoto T, Tezuka Y. Programmed polymer folding with periodically positioned tetrafunctional telechelic precursors by cyclic ammonium salt units as nodal points. J Am Chem Soc. 2019;141:7526–36.
Wagner HL. The Mark–Houwink–Sakurada equation for the viscosity of atactic polystyrene. J Phys Chem Ref Data. 1985;14:1101–6.
Liénard R, De Winter J, Coulembier O. Cyclic polymers: advances in their synthesis, properties, and biomedical applications. J Polym Sci. 2020;58:1481–502.
Chen C, Weil T. Cyclic polymers: synthesis, characteristics, and emerging applications. Nanoscale Horiz. 2022;7:1121–35.
Haque FM, Grayson SM. The synthesis, properties and potential applications of cyclic polymers. Nat Chem. 2020;12:433–44.
Kricheldorf HR, Lee SR. Polylactones. 40. Nanopretzels by macrocyclic polymerization of lactones via a spirocyclic tin initiator derived from pentaerythritol. Macromolecules. 1996;29:8689–95.
Zhang Z, Nie X, Wang F, Chen G, Huang WQ, Xia L, et al. Rhodanine-based Knoevenagel reaction and ring-opening polymerization for efficiently constructing multicyclic polymers. Nat Commun. 2020;11:3654.
Chen J, Li H, Zhang H, Liao X, Han H, Zhang L, et al. Blocking-cyclization technique for precise synthesis of cyclic polymers with regulated topology. Nat Commun. 2018;9:5310.
Lonsdale DE, Monteiro MJ. Various polystyrene topologies built from tailored cyclic polystyrene via CuAAC reactions. Chem Commun. 2010;46:7945–7.
Ko YS, Yamamoto T, Tezuka Y. Click construction of spiro- and bridged-quatrefoil polymer topologies with kyklo-telechelics having an azide group. Macromol Rapid Commun. 2014;35:412–6.
Sugai N, Heguri H, Ohta K, Meng Q, Yamamoto T, Tezuka Y. Effective click construction of bridged-and spiro-multicyclic polymer topologies with tailored cyclic prepolymers (kyklo-telechelics). J Am Chem Soc. 2010;132:14790–802.
Jeong J, Kim K, Lee R, Lee S, Kim H, Jung H, et al. Preparation and analysis of bicyclic polystyrene. Macromolecules. 2014;47:3791–6.
Lee T, Oh J, Jeong J, Jung H, Huh J, Chang T, et al. Shaped and cage-shaped cyclic polystyrenes. Macromolecules. 2016;49:3672–80.
Sugai N, Heguri H, Yamamoto T, Tezuka Y. A programmed polymer folding: click and clip construction of doubly fused tricyclic and triply fused tetracyclic polymer topologies. J Am Chem Soc. 2011;133:19694–7.
Tezuka Y. Topological polymer chemistry for designing multicyclic macromolecular architectures. Polym J. 2012;44:1159–69.
Zhang Y, Wu Y, Zhao Y, Zhang L, Zhang K. Versatile bimolecular ring-closure method for cage-shaped polymers. Macromolecules. 2021;54:6901–10.
Shi GY, Pan CY. Synthesis of well-defined figure-of-eight-shaped polymers by a combination of ATRP and click chemistry. Macromol Rapid Commun. 2008;29:1672–8.
Bukowski C, Zhang T, Riggleman RA, Crosby AJ. Load-bearing entanglements in polymer glasses. Sci Adv. 2021;7:eabg9763.
Gauthier-Jaques M, Theato P. Synergy of macrocycles and macromolecular topologies: an efficient [34]Triazolophane-based synthesis of cage-shaped polymers. ACS Macro Lett. 2020;9:700–5.
Satoh Y, Matsuno H, Yamamoto T, Tajima K, Isono T, Satoh T. Synthesis of well-defined three- and four-armed cage-shaped polymers via ‘topological conversion’ from trefoil- and quatrefoil-shaped polymers. Macromolecules. 2017;50:97–106.
Isono T, Sasamori T, Honda K, Mato Y, Yamamoto T, Tajima K, et al. Multicyclic polymer synthesis through controlled/living cyclopolymerization of α,ω-Dinorbornenyl-Functionalized macromonomers. Macromolecules. 2018;51:3855–64.
Ebii Y, Mato Y, Li F, Tajima K, Yamamoto T, Isono T, et al. Cyclopolymerization: a versatile approach toward multicyclic polystyrene and polystyrene-containing multicyclic copolymers. Polym Chem. 2023;14:3099–109.
Radzinski SC, Foster JC, Chapleski RC, Troya D, Matson JB. Bottlebrush polymer synthesis by ring-opening metathesis polymerization: the significance of the anchor group. J Am Chem Soc. 2016;138:6998–7004.
Xia Y, Kornfield JA, Grubbs RH. Efficient synthesis of narrowly dispersed brush polymers via living ring-opening metathesis polymerization of macromonomers. Macromolecules. 2009;42:3761–6.
Li A, Li Z, Zhang S, Sun G, Policarpio DM, Wooley KL. Synthesis and direct visualization of dumbbell-shaped molecular brushes. ACS Macro Lett. 2012;1:241–5.
Makiguchi K, Satoh T, Kakuchi T. Diphenyl phosphate as an efficient cationic organocatalyst for controlled/living ring-opening polymerization of δ-valerolactone and ε-caprolactone. Macromolecules. 2011;44:1999–2005.
Mato Y, Honda K, Tajima K, Yamamoto T, Isono T, Satoh T. A versatile synthetic strategy for macromolecular cages: intramolecular consecutive cyclization of star-shaped polymers. Chem Sci. 2019;10:440–6.
Mato Y, Honda K, Ree BJ, Tajima K, Yamamoto T, Deguchi T, et al. Programmed folding into spiro-multicyclic polymer topologies from linear and star-shaped chains. Commun Chem. 2020;3:97.
Mato Y, Sudo M, Marubayashi H, Ree BJ, Tajima K, Yamamoto T, et al. Densely arrayed cage-shaped polymer topologies synthesized via cyclopolymerization of star-shaped macromonomers. Macromolecules. 2021;54:9079–90.
Li Z, Zhang KE, Ma J, Cheng C, Wooley KL. Facile syntheses of cylindrical molecular brushes by a sequential RAFT and ROMP ‘Grafting-Through’ methodology. J Polym Sci A Polym Chem. 2009;47:5557–63.
Hsu HP, Paul W, Rathgeber S, Binder K. Characteristic length scales and radial monomer density profiles of molecular bottle-brushes: simulation and experiment. Macromolecules. 2010;43:1592–601.
Li Z, Ma J, Lee NS, Wooley KL. Dynamic cylindrical assembly of triblock copolymers by a hierarchical process of covalent and supramolecular interactions. J Am Chem Soc. 2011;133:1228–31.
Pesek SL, Li X, Hammouda B, Hong K, Verduzco R. Small-angle neutron scattering analysis of bottlebrush polymers prepared via grafting-through polymerization. Macromolecules. 2013;46:6998–7005.
Fox TG, Flory PJ. Second-order transition temperatures and related properties of polystyrene. I. Influence of molecular weight. J Appl Phys. 1950;21:581–91.
Gao L, Oh J, Tu Y, Chang T, Li CY. Glass transition temperature of cyclic polystyrene and the linear counterpart contamination effect. Polymer (Guildf). 2019;170:198–203.
Ebe M, Soga A, Fujiwara K, Ree BJ, Marubayashi H, Hagita K, et al. Rotaxane formation of multicyclic polydimethylsiloxane in a silicone network: a step toward constructing “macro-rotaxanes” from high-molecular-weight axle and wheel components. Angew Chem Int Ed Engl. 2023;62:e202304493.
Acknowledgements
This work was financially supported by a MEXT Grant-in-Aid for Challenging Exploratory Research (19K22209 and 24K21788), JST CREST (JPMJCR19T4), the Eno Scientific Foundation, Tokyo Ohka Foundation for the Promotion of Science and Technology, and the Sumitomo Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ebii, Y., Ebe, M., Li, F. et al. Multicyclic polymer synthesis via a consecutive cyclization approach. Polym J 57, 1295–1311 (2025). https://doi.org/10.1038/s41428-025-01078-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41428-025-01078-w