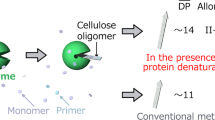
Abstract
Protein adsorption onto surfaces is a critical process in biomaterial science, influencing subsequent biological responses. Understanding this phenomenon is key to designing materials with controlled interactions for biomedical applications. Here, we report controllable protein adsorption onto crystalline lamellar assemblies of carboxylated cello-oligosaccharides synthesized via cellodextrin phosphorylase-catalyzed oligomerization. These assemblies, featuring a terminal carboxy group linked by alkyl chains, possessed a negative surface charge, the magnitude of which depended on alkyl linker length, pH, and ionic strength, as confirmed by zeta potential measurements. Under varying pH and ionic strength conditions, we observed significant adsorption of basic proteins, which increased with longer alkyl linkers and lower ionic strength. Although acidic protein adsorption at acidic pH was minimal under high ionic strength, we notably found that acidic proteins were adsorbed onto negatively charged assemblies under low ionic strength. Our results demonstrate that electrostatic interactions primarily govern protein adsorption on these assemblies, enabling controllable protein adsorption through the adjustment of their surface properties and solution conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ogoshi T. Supramolecular assemblies and polymer recognition based on polygonal and pillar-shaped macrocycles “pillar[n]arenes. Polym J. 2023;55:1247–60.
Masuda T. Design of functional soft interfaces with precise control of the polymer architecture. Polym J. 2024;56:643–52.
Uchida N. Design of supramolecular nanosheets for drug delivery applications. Polym J. 2023;55:829–36.
Inaba H. Construction of functional microtubules and artificial motile systems based on peptide design. Polym J. 2023;55:1261–74.
Murai K. Development of peptide–inorganic hybrid materials based on biomineralization and their functional design based on structural controls. Polym J. 2023;55:817–27.
Nishimura S, Tanaka M. The intermediate water concept for pioneering polymeric biomaterials: a review and update. Bull Chem Soc Jpn. 2023;96:1052–70.
Hattori T. Colloidal titration: from the perspective of stability constants between oppositely charged polyelectrolytes. Bull Chem Soc Jpn. 2024;97:uoae044.
Roy B, Govindaraju T. Enzyme-mimetic catalyst architectures: the role of second coordination sphere in catalytic activity. Bull Chem Soc Jpn. 2024;97:bcsj.20230224.
Miyamoto T, Numata K. Advancing biomolecule delivery in plants: harnessing synthetic nanocarriers to overcome multiscale barriers for cutting-edge plant bioengineering. Bull Chem Soc Jpn. 2023;96:1026–44.
Nilsson PH, Ekdahl KN, Magnusson PU, Qu H, Iwata H, Ricklin D, et al. Autoregulation of thromboinflammation on biomaterial surfaces by a multicomponent therapeutic coating. Biomaterials. 2013;34:985–94.
Sivaraman B, Latour RA. The relationship between platelet adhesion on surfaces and the structure versus the amount of adsorbed fibrinogen. Biomaterials. 2010;31:832–9.
Wei Q, Becherer T, Angioletti-Uberti S, Dzubiella J, Wischke C, Neffe AT, et al. Protein interactions with polymer coatings and biomaterials. Angew Chem Int Ed. 2014;53:8004–31.
Firkowska-Boden I, Zhang X, Jandt KD. Controlling protein adsorption through nanostructured polymeric surfaces. Adv Health Mater. 2018;7:1700995.
Li J, Han Q, Zhang Y, Zhang W, Dong M, Besenbacher F, et al. Optical regulation of protein adsorption and cell adhesion by photoresponsive GaN nanowires. ACS Appl Mater Interfaces. 2013;5:9816–22.
Tang J, Qiu Y, Li Z. Osteopontin facilitated dental pulp cell adhesion and differentiation: a laboratory investigation. ACS Appl Bio Mater. 2025;8:1320–9.
Kubiak-Ossowska K, Tokarczyk K, Jachimska B, Mulheran PA. Bovine serum albumin adsorption at a silica surface explored by simulation and experiment. J Phys Chem B. 2017;121:3975–86.
Kubiak-Ossowska K, Mulheran PA. Mechanism of hen egg white lysozyme adsorption on a charged solid surface. Langmuir. 2010;26:15954–65.
Miyagawa A, Hagiya K, Nagatomo S, Nakatani K. Protein adsorption on carboxy-functionalized microparticles revealed by zeta potential and absorption spectroscopy measurements. Bull Chem Soc Jpn. 2023;96:759–65.
Thiramanas R, Wongngam Y, Supanakorn G, Polpanich D. BSA adsorption on titanium dioxide nanoparticle surfaces for controlling their cellular uptake in skin cells. ACS Appl Bio Mater. 2024;7:1713–22.
Moerz ST, Huber P. pH-dependent selective protein adsorption into mesoporous silica. J Phys Chem C. 2015;119:27072–9.
Meissner J, Prause A, Bharti B, Findenegg GH. Characterization of protein adsorption onto silica nanoparticles: influence of pH and ionic strength. Colloid Polym Sci. 2015;293:3381–91.
Kubiak-Ossowska K, Jachimska B, Mulheran PA. How negatively charged proteins adsorb to negatively charged surfaces: a molecular dynamics study of BSA adsorption on silica. J Phys Chem B. 2016;120:10463–8.
Salgın S, Takaç S, Özdamar TH. Adsorption of bovine serum albumin on polyether sulfone ultrafiltration membranes: determination of interfacial interaction energy and effective diffusion coefficient. J Membr Sci. 2006;278:251–60.
Yu Q, Chen H, Zhang Y, Yuan L, Zhao T, Li X, et al. pH-reversible, high-capacity binding of proteins on a substrate with nanostructure. Langmuir. 2010;26:17812–5.
Hober S, Nord K, Linhult M. Protein A chromatography for antibody purification. J Chromatogr B. 2007;848:40–7.
Jung Y-D, Khan M, Park S-Y. Fabrication of temperature- and pH-sensitive liquid-crystal droplets with PNIPAM-b-LCP and SDS coatings by microfluidics. J Mater Chem B. 2014;2:4922–8.
Klemm D, Heublein B, Fink H-P, Bohn A. Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed. 2005;44:3358–93.
Li T, Chen C, Brozena AH, Zhu JY, Xu L, Driemeier C, et al. Developing fibrillated cellulose as a sustainable technological material. Nature. 2021;590:47–56.
Heise K, Kontturi E, Allahverdiyeva Y, Tammelin T, Linder MB, Nonappa, et al. Nanocellulose: recent fundamental advances and emerging biological and biomimicking applications. Adv Mater. 2021;33:2004349.
Klemm D, Kramer F, Moritz S, Lindström T, Ankerfors M, Gray D, et al. Nanocelluloses: a new family of nature-based materials. Angew Chem Int Ed. 2011;50:5438–66.
Habibi Y, Lucia LA, Rojas OJ. Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem Rev. 2010;110:3479–500.
Tardy BL, Mattos BD, Otoni CG, Beaumont M, Majoinen J, Kämäräinen T, et al. Deconstruction and reassembly of renewable polymers and biocolloids into next generation structured materials. Chem Rev. 2021;121:14088–188.
Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J. Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev. 2011;40:3941–94.
Barhoum A, Jeevanandam J, Rastogi A, Samyn P, Boluk Y, Dufresne A, et al. Plant celluloses, hemicelluloses, lignins, and volatile oils for the synthesis of nanoparticles and nanostructured materials. Nanoscale. 2020;12:22845–90.
Shi Y, Jiao H, Sun J, Lu X, Yu S, Cheng L, et al. Functionalization of nanocellulose applied with biological molecules for biomedical application: a review. Carbohydr Polym. 2022;285:119208.
Ong X-R, Chen AX, Li N, Yang YY, Luo H-K. Nanocellulose: recent advances toward biomedical applications. Small Sci. 2023;3:2200076.
Hata Y, Serizawa T. Nanoarchitectonics of cello-oligosaccharides: a route toward artificial nanocelluloses. Adv Colloid Interface Sci. 2025;336:103361.
Hiraishi M, Igarashi K, Kimura S, Wada M, Kitaoka M, Samejima M. Synthesis of highly ordered cellulose II in vitro using cellodextrin phosphorylase. Carbohydr Res. 2009;344:2468–73.
Sletten ET, Fittolani G, Hribernik N, Dal Colle MCS, Seeberger PH, Delbianco M. Phosphates as assisting groups in glycan synthesis. ACS Cent Sci. 2024;10:138–42.
Djalali S, Yadav N, Delbianco M. Towards glycan foldamers and programmable assemblies. Nat Rev Mater. 2024;9:190–201.
Hata Y, Serizawa T. Self-assembly of cellulose for creating green materials with tailor-made nanostructures. J Mater Chem B. 2021;9:3944–66.
Hata Y, Serizawa T. Robust gels composed of self-assembled cello-oligosaccharide networks. Bull Chem Soc Jpn. 2021;94:2279–89.
Serizawa T, Yamaguchi S, Sugiura K, Marten R, Yamamoto A, Hata Y, et al. Antibacterial synthetic nanocelluloses synergizing with a metal-chelating agent. ACS Appl Bio Mater. 2024;7:246–55.
Mizuuchi Y, Hata Y, Sawada T, Serizawa T. Surface-mediated self-assembly of click-reactive cello-oligosaccharides for fabricating functional nonwoven fabrics. Sci Technol Adv Mater. 2024;25:2311052.
Suehiro F, Hata Y, Sawada T, Serizawa T. Freeze-dryable, stable, and click-reactive nanoparticles composed of cello-oligosaccharides for biomolecular sensing. ACS Appl Bio Mater. 2024;7:4007–16.
Sugiura K, Sawada T, Hata Y, Tanaka H, Serizawa T. Distinguishing anti-PEG antibodies by specificity for the PEG terminus using nanoarchitectonics-based antibiofouling cello-oligosaccharide platforms. J Mater Chem B. 2024;12:650–7.
Sugiura K, Saito M, Sawada T, Tanaka H, Serizawa T. Cellodextrin phosphorylase-catalyzed single-process production and superior mechanical properties of organic–inorganic hybrid hydrogels composed of surface-carboxylated synthetic nanocelluloses and hydroxyapatite. ACS Sustain Chem Eng. 2022;10:13484–94.
Nohara T, Sawada T, Tanaka H, Serizawa T. Templated synthesis of gold nanoparticles on surface-aminated 2D cellulose assemblies. Bull Chem Soc Jpn. 2019;92:982–8.
Nohara T, Sawada T, Tanaka H, Serizawa T. Enzymatic synthesis and protein adsorption properties of crystalline nanoribbons composed of cellulose oligomer derivatives with primary amino groups. J Biomater Sci Polym Ed. 2017;28:925–38.
Serizawa T, Yamaguchi S, Amitani M, Ishii S, Tsuyuki H, Tanaka Y, et al. Alkyl chain length-dependent protein nonadsorption and adsorption properties of crystalline alkyl β-celluloside assemblies. Colloids Surf B. 2022;220:112898.
Langan P, Nishiyama Y, Chanzy H. X-ray structure of mercerized cellulose II at 1 Å resolution. Biomacromolecules. 2001;2:410–6.
Lvov Y, Ariga K, Ichinose I, Kunitake T. Assembly of multicomponent protein films by means of electrostatic layer-by-layer adsorption. J Am Chem Soc. 1995;117:6117–23.
Alves JEF, Lucena MLC, de Souza Lucena AE, das Merces AAD, de Azevedo RDS, Sousa GLS, et al. SMV. A simple method for obtaining human albumin and its use for in vitro interaction assays with indole-thiazole and indole-thiazolidinone derivatives. Int J Biol Macromol. 2021;192:126–37.
Serizawa T, Maeda T, Sawada T. Neutralization-induced self-assembly of cellulose oligomers into antibiofouling crystalline nanoribbon networks in complex mixtures. ACS Macro Lett. 2020;9:301–5.
Jiang X, Weise S, Hafner M, Röcker C, Zhang F, Parak WJ, et al. Quantitative analysis of the protein corona on FePt nanoparticles formed by transferrin binding. J R Soc Interface. 2009;7:S5–13.
Wangkam T, Yodmongkol S, Disrattakit J, Sutapun B, Amarit R, Somboonkaew A, et al. Adsorption of bovine serum albumin (BSA) on polystyrene (PS) and its acid copolymer. Curr Appl Phys. 2012;12:44–52.
He XM, Carter DC. Atomic structure and chemistry of human serum albumin. Nature. 1992;358:209–15.
Reynolds EC, Wong A. Effect of adsorbed protein on hydroxyapatite zeta potential and Streptococcus mutans adherence. Infect Immun. 1983;39:1285–90.
Hanamura M, Sawada T, Serizawa T. In-paper self-assembly of cellulose oligomers for the preparation of all-cellulose functional paper. ACS Sustain Chem Eng. 2021;9:5684–92.
Hata Y, Miyazaki H, Okamoto S, Serizawa T, Nakamura S. Nanospiked cellulose gauze that attracts bacteria with biomolecules for reducing bacterial load in burn wounds. Nano Lett. 2025;25:1177–84.
Wang Y, Nor YA, Song H, Yang YN, Xu C, Yu MH, et al. Small-sized and large-pore dendritic mesoporous silica nanoparticles enhance antimicrobial enzyme delivery. J Mater Chem B. 2016;4:2646–53.
Anastas PT, Rodriguez A, de Winter TM, Coish P, Zimmerman JB. A review of immobilization techniques to improve the stability and bioactivity of lysozyme. Green Chem Lett Rev. 2021;14:302–38.
Acknowledgements
We thank Professor Toshiki Sawada (Institute of Science Tokyo) for his valuable contributions through helpful advice on the experiments and insightful discussions. The authors thank Professor Shingo Nakamura, Professor Hiromi Miyazaki, and Dr. Kazunori Morohoshi (National Defense Medical College Research Institute) for zeta potential measurements. We are grateful for the financial support to TS from a Grant-in-Aid for Scientific Research on Innovative Areas (Aquatic Functional Materials) (JP22H04528) from the Ministry of Education, Culture, Sports, Science and Technology, Japan and a Grant-in-Aid for Scientific Research (B) (JP24K01548) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sugiura, K., Matsunami, A., Hata, Y. et al. Electrostatic control of protein adsorption onto negatively charged crystalline cello-oligosaccharide assemblies. Polym J (2025). https://doi.org/10.1038/s41428-025-01104-x
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41428-025-01104-x