Abstract
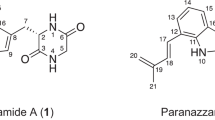
During our screening program for new potentiators of amphotericin B activity against Candida albicans, shodoamides A to C (1–3) were isolated from a culture broth of the fungus Pseudophialophora sp. BF-0158 fermented under shaking conditions. A known congener named shodoamide D (4) in this paper was obtained from a culture broth of the BF-0158 strain fermented under static conditions. The structures of 1–4 were assigned based on spectroscopic analyses, including NMR and MS, and were found to have a common N-(2´,3´,4´-trihydroxybutyl)-6-methyl-2,4-tetradecadienamide structure. Compounds 1–3 exhibited no antifungal activity, but they induced up to 32-fold increases in amphotericin B activity against C. albicans by a microdilution method.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
03 March 2025
The original online version of this article was revised: The authors of the above article noted an error in the publication of this paper, whereby the incorrect data were used for the chemical shifts of hydroxyl groups.
In the 'Structural elucidation of shodoamide' section on page 580, right-hand column, please replace the chemical shifts of 12-OH for 2 and 13-OH for 3 with the following corrected values:
12-OH: δ 4.20 (previously reported as δ 4.43)
13-OH: δ 4.28 (previously reported as δ 4.43)
This revision is based on studies of the total synthesis of shodoamide C.
Additionally, the corrected data in Table 2 are shown below.
The original article has been corrected.
13 March 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41429-025-00814-x
References
Trejo WH, Bennett RE. Streptomyces nodosus sp. n., the amphotericin-producing organism. J Bacteriol. 1963;85:436–9.
Uchida R, Kondo A, Yagi A, Nonaka K, Masuma R, Kobayashi K, Tomoda H. Simpotentin, a new potentiator of amphotericin B activity against Candida albicans, produced by Simplicillium minatense FKI-4981. J Antibiot. 2019;72:134–40.
Ohtawa M, Shimizu E, Saito A, Sakamoto S, Waki A, Kondo A, Yagi A, Uchida R, Tomoda H, Nagamitsu T. Total synthesis and absolute configuration of simpotentin, a potentiator of amphotericin B activity. Org Lett. 2019;21:5596–9.
Fukuda T, Nagai K, Yagi A, Kobayashi K, Uchida R, Yasuhara T, Tomoda H. Nectriatide, a potentiator of amphotericin B activity from Nectriaceae sp. BF-0114. J Nat Prod. 2019;82:2673–81.
Yagi A, Uchida R, Kobayashi K, Tomoda H. Polyketide glycosides phialotides A to H, new potentiators of amphotericin B activity, produced by Pseudophialophora sp. BF-0158. J Antibiot. 2020;73:211–23.
Yagi A, Yamaguchi Y, Kawasaki K, Usui E, Yamazaki H, Uchida R. New piericidin rhamnosides as potentiators of amphotericin B activity against Candida albicans produced by actinomycete strain TMPU-A0287. J Antibiot. 2023;76:65–74.
Bolessa EA, Schwartz RE, Bills GF, Giacobbe RA, Pelaez PF, Cabello AA, Diez MT, Martin FI, Vincente PF. Antifungal agent formulations containing alkadienes. United States, US5461071 A 1995-10-24.
Luo J, Walsh E, Blystone D, Zhang N. Five new Pseudophialophora species from grass roots in the oligotrophic pine barrens ecosystem. Fungal Biol. 2015;119:1205–15.
Luo J, Walsh E, Zhang N. Four new species in Magnaporthaceae from grass roots in New Jersey Pine Barrens. Mycologia. 2014;106:580–8.
Zhu JN, Yu YJ, Dai MD, Zeng YL, Lu XJ, Wang L, Liu XH, Su ZZ, Lin FC. A New Species in Pseudophialophora from wild rice and beneficial potential. Front Microbiol. 2022;13:845104.
Ishijima H, Uchida R, Ohtawa M, Kondo A, Nagai K, Shima K, Nonaka K, Masuma R, Iwamoto S, Onodera H, Nagamitsu T, Tomoda H. Simplifungin and valsafungins, antifungal antibiotics of fungal origin. J Org Chem. 2016;81:7373–83.
Clinical and Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of yeasts. 3rd edition; CLSI document M27-A3. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
Clinical and Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard CLSI document M38-A2. 3rd edition. Wayne, PA: Clinical and Laboratory Standards Institute; 2008
Acknowledgements
We express our thanks to Dr Kenichiro Nagai and Ms Noriko Sato of the School of Pharmacy, Kitasato University, for measuring NMR and mass spectra.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The authors of the above article noted an error in the publication of this paper, whereby the incorrect data were used for the chemical shifts of hydroxyl groups.
In the 'Structural elucidation of shodoamide' section on page 580, right-hand column, please replace the chemical shifts of 12-OH for 2 and 13-OH for 3 with the following corrected values:
12-OH: δ 4.20 (previously reported as δ 4.43)
13-OH: δ 4.28 (previously reported as δ 4.43)
This revision is based on studies of the total synthesis of shodoamide C.
Additionally, the corrected data in Table 2 are shown below.
The original article has been corrected.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yagi, A., Kashima, M., Ishijima, H. et al. New potentiators of amphotericin B activity, shodoamides A to C produced by Pseudophialophora sp. BF-0158. J Antibiot 76, 579–584 (2023). https://doi.org/10.1038/s41429-023-00642-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41429-023-00642-x