Abstract
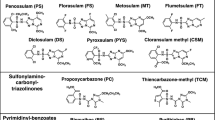
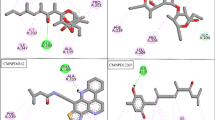
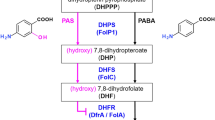
Acetohydroxyacid synthase (AHAS), exclusively present in microorganisms and plants, is a promising target for several herbicides due to its catalytic role in the branched-chain amino acid biosynthetic pathway. Previous studies have shown that K13787, a pyrazolopyrimidine sulfonamide AHAS inhibitor, was moderately effective against pulmonary infection caused by M. tuberculosis and nontuberculous mycobacteria (NTM). In this study, we synthesized various structural derivatives of K13787 based on the molecular docking studies and assessed their MICs against mycobacteria species. Among the synthetic compounds screened, K13787, along with KNT2077 and KNT2099, exhibited inhibitory efficacy against M. avium and M. abscessus, including CLR-resistant NTM species. Notably, these compounds displayed a synergistic effect (FIC ≤ 0.5) when combined with CLR against M. avium and M. abscessus. Our findings suggest that these newly identified AHAS-targeted compounds hold promise as lead candidates for novel antimycobacterial agents against NTM infections. Considering the structure-activity relationship, K13787, KNT2077, and KTN2099 emerge as potential treatments for NTM species.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gopalaswamy R, Shanmugam S, Mondal R, Subbian S. Of tuberculosis and non-tuberculous mycobacterial infections - a comparative analysis of epidemiology, diagnosis and treatment. J Biomed Sci. 2020;27:74.
Ratnatunga CN, Lutzky VP, Kupz A, Doolan DL, Reid DW, Field M, et al. The rise of non-tuberculosis mycobacterial lung disease. Front Immunol. 2020;11:303.
Kim BG, Jhun BW, Kim H, Kwon OJ. Treatment outcomes of Mycobacterium avium complex pulmonary disease according to disease severity. Sci Rep. 2022;12:1970.
Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ, Andrejak C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis. 2020;71:905–13.
Taylor JL, Palmer SM. Mycobacterium abscessus chest wall and pulmonary infection in a cystic fibrosis lung transplant recipient. J Heart Lung Transpl. 2006;25:985–8.
Huh HJ, Kim SY, Shim HJ, Kim DH, Yoo IY, Kang OK et al. GenoType NTM-DR performance evaluation for identification of mycobacterium avium complex and mycobacterium abscessus and determination of clarithromycin and amikacin resistance. J Clin Microbiol. 2019;57:e00516-19.
Ruth MM, Sangen JJN, Remmers K, Pennings LJ, Svensson E, Aarnoutse RE, et al. A bedaquiline/clofazimine combination regimen might add activity to the treatment of clinically relevant non-tuberculous mycobacteria. J Antimicrob Chemother. 2019;74:935–43.
Thiour-Mauprivez C, Martin-Laurent F, Calvayrac C, Barthelmebs L. Effects of herbicide on non-target microorganisms: towards a new class of biomarkers? Sci Total Environ. 2019;684:314–25.
Sohn H, Lee KS, Ko YK, Ryu JW, Woo JC, Koo DW, et al. In vitro and ex vivo activity of new derivatives of acetohydroxyacid synthase inhibitors against Mycobacterium tuberculosis and non-tuberculous mycobacteria. Int J Antimicrob Agents. 2008;31:567–71.
Choi KJ, Yu YG, Hahn HG, Choi JD, Yoon MY. Characterization of acetohydroxyacid synthase from Mycobacterium tuberculosis and the identification of its new inhibitor from the screening of a chemical library. FEBS Lett. 2005;579:4903–10.
Gokhale K, Tilak B. Mechanisms of bacterial acetohydroxyacid synthase (AHAS) and specific inhibitors of Mycobacterium tuberculosis AHAS as potential drug candidates against tuberculosis. Curr Drug Targets. 2015;16:689–99.
Saxena S, Spaink HP, Forn-Cuni G. Drug resistance in nontuberculous mycobacteria: mechanisms and models. Biology (Basel). 2021;10:96.
Song CH, Kim HJ, Lim HJ et al. Novel compound and pharmaceutical composition for treating Mycobacterium tuberculosis ir nontuberculous Mycobacterium infection comprising the same. KR 20230106356. 2023.
Garcia MD, Chua SMH, Low YS, Lee YT, Agnew-Francis K, Wang JG, et al. Commercial AHAS-inhibiting herbicides are promising drug leads for the treatment of human fungal pathogenic infections. Proc Natl Acad Sci USA. 2018;115:E9649–E9658.
Sun Y, Zhang Y et al. Preparation method of diclosulam. CN 106905323. 2017.
Institute CaLS. M62: Performance Standards for Susceptibility Testing of Mycobacteria,Nocardia spp., and Other Aerobic Actinomycetes. CLSI: Wayne, PA, 2018.
Parvekar P, Palaskar J, Metgud S, Maria R, Dutta S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater Investig Dent. 2020;7:105–9.
Son SH, Lee J, Cho SN, Choi JA, Kim J, Nguyen TD, et al. Herp regulates intracellular survival of Mycobacterium tuberculosisH37Ra in macrophages by regulating reactive oxygen species-mediated autophagy. mBio. 2023;14:e0153523.
Magi G, Marini E, Facinelli B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front Microbiol. 2015;6:165.
Bonapace CR, Bosso JA, Friedrich LV, White RL. Comparison of methods of interpretation of checkerboard synergy testing. Diagn Microbiol Infect Dis. 2002;44:363–6.
Konate K, Mavoungou JF, Lepengue AN, Aworet-Samseny RR, Hilou A, Souza A, et al. Antibacterial activity against beta- lactamase producing Methicillin and Ampicillin-resistants Staphylococcus aureus: Fractional Inhibitory Concentration Index (FICI) determination. Ann Clin Microbiol Antimicrob. 2012;11:18.
Eliopoulos, GM, RC Moellering. Antibiotics combinations, p.432-492. In V. Lorian (ed.), Antibiotics in laboratory medicine, 3rd ed. The Williams & Wilkins Co., Baltomore, MD. 1991.
Zhao J, Zhang Z, Xue Y, Wang G, Cheng Y, Pan Y, et al. Anti-tumor macrophages activated by ferumoxytol combined or surface-functionalized with the TLR3 agonist poly (I : C) promote melanoma regression. Theranostics. 2018;8:6307–21.
Fukuda H, Ohashi Y. A guideline for reporting results of statistical analysis in Japanese Journal of Clinical Oncology. Jpn J Clin Oncol. 1997;27:121–7.
Stout JE, Koh WJ, Yew WW. Update on pulmonary disease due to non-tuberculous mycobacteria. Int J Infect Dis. 2016;45:123–34.
Lee YT, Cui CJ, Chow EW, Pue N, Lonhienne T, Wang JG, et al. Sulfonylureas have antifungal activity and are potent inhibitors of Candida albicans acetohydroxyacid synthase. J Med Chem. 2013;56:210–9.
Pan L, Jiang Y, Liu Z, Liu XH, Liu Z, Wang G, et al. Synthesis and evaluation of novel monosubstituted sulfonylurea derivatives as antituberculosis agents. Eur J Med Chem. 2012;50:18–26.
Kreisberg JF, Ong NT, Krishna A, Joseph TL, Wang J, Ong C, et al. Growth inhibition of pathogenic bacteria by sulfonylurea herbicides. Antimicrob Agents Chemother. 2013;57:1513–7.
Meng FF, Shang MH, Wei W, Yu ZW, Liu JL, Li ZM et al. Novel sulfonylurea derivatives as potential antimicrobial agents: chemical synthesis, biological evaluation, and computational study. Antibiotics (Basel). 2023;12:323.
Ovung A, Bhattacharyya J. Sulfonamide drugs: structure, antibacterial property, toxicity, and biophysical interactions. Biophys Rev. 2021;13:259–72.
Chio LC, Bolyard LA, Nasr M, Queener SF. Identification of a class of sulfonamides highly active against dihydropteroate synthase form Toxoplasma gondii, Pneumocystis carinii, and Mycobacterium avium. Antimicrob Agents Chemother. 1996;40:727–33.
McFarland MM, Zach SJ, Wang X, Potluri LP, Neville AJ, Vennerstrom JL, et al. Review of experimental compounds demonstrating anti-toxoplasma activity. Antimicrob Agents Chemother. 2016;60:7017–34.
Acknowledgements
TDN, JAC: Designed the study, Performance the majority of the experiments, Analyzed the data, Validation, Writing—original draft. H-JL, CHC: Providing the synthetics compounds and expert technical assistance. JL: Methodology, Validation. S-HS: Data curation, Methodology. JK: Methodology, Validation. DS: Investigation, Formal analysis. H-JK: Conceptualization, Supervision, Writing—review and editing. C-HS: Designed the study, Supervision, Project administration, Funding acquisition, Writing—review and editing. All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2022R1C1C2011153). The funders had no role in study design, data collection and analysis decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Chungnam National University (Daejeon, Korea, CNU-00907). Animal experiments were performed in accordance with Korean Food and Drug Administration guidelines.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nguyen, T.D., Choi, JA., Lim, HJ. et al. Inhibitors of acetohydroxyacid synthase as promising agents against non-tuberculous mycobacterial diseases. J Antibiot 78, 181–189 (2025). https://doi.org/10.1038/s41429-024-00799-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41429-024-00799-z